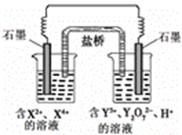
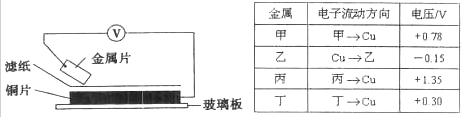
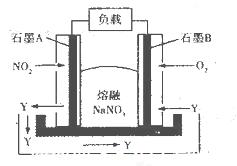
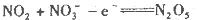��Ŀ����
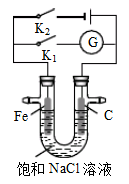
�״��ڴ����������ṩ����(H+)�͵��ӣ����Ӿ����·�����Ӿ��ڵ�·������һ����������Ӧ������ܷ�ӦʽΪ2CH3OH+3O2 2CO2+4H2O�����й��ڸõ�صķ������ټ״��Ǹ�����H+���ƶ��۸�����Ӧ��CH3OH-6e-+H2O=CO2+6H+ ��1 mol CH3OH��ȫ��Ӧת��12 mol���� ��������Ӧ��O2+4e-+2H2O==4OH-�õ�صĵ������Һ�����Ǽ�Һ��������ȷ����
2CO2+4H2O�����й��ڸõ�صķ������ټ״��Ǹ�����H+���ƶ��۸�����Ӧ��CH3OH-6e-+H2O=CO2+6H+ ��1 mol CH3OH��ȫ��Ӧת��12 mol���� ��������Ӧ��O2+4e-+2H2O==4OH-�õ�صĵ������Һ�����Ǽ�Һ��������ȷ����
 2CO2+4H2O�����й��ڸõ�صķ������ټ״��Ǹ�����H+���ƶ��۸�����Ӧ��CH3OH-6e-+H2O=CO2+6H+ ��1 mol CH3OH��ȫ��Ӧת��12 mol���� ��������Ӧ��O2+4e-+2H2O==4OH-�õ�صĵ������Һ�����Ǽ�Һ��������ȷ����
2CO2+4H2O�����й��ڸõ�صķ������ټ״��Ǹ�����H+���ƶ��۸�����Ӧ��CH3OH-6e-+H2O=CO2+6H+ ��1 mol CH3OH��ȫ��Ӧת��12 mol���� ��������Ӧ��O2+4e-+2H2O==4OH-�õ�صĵ������Һ�����Ǽ�Һ��������ȷ����| A���٢ڢ� | B���ڢۢܢ� | C���� | D���٢� |
D
������������ݵ�ط�Ӧ����ʽ�жϣ��״�����������Ӧ�����Լ״��Ǹ�����������Ӧ��CH3OH-6e-+H2O=CO2+6H+ ����1 mol CH3OH��ȫ��Ӧת��6 mol���ӣ���������������Ϊ�״��ڴ����������ṩ����(H+)�͵��ӣ������������ﲻ������OH-��������Ӧ�ǣ�O2+4e-+4H+==2H2O���������Һ�������Ǽ�Һ����ʹ��Һ��ԭ����е�����������������������ȷ��ѡD��

��ϰ��ϵ�д�
�����Ŀ