��Ŀ����
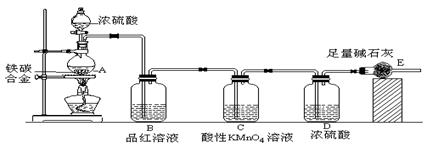
��6�֣���֪ijȼ�Ϻ�̼���⡢������Ԫ�أ�Ϊ�˲ⶨ��ȼ�ϵ���ɣ�����ȼ�Ϸ�������������ȼ�գ���ʹ������CO2��H2O������ʣ���O2ȫ��ͨ����ͼ��ʾ��װ�ã��õ����±����е�ʵ�����ݣ��������ɵ�����ȫ�������գ���
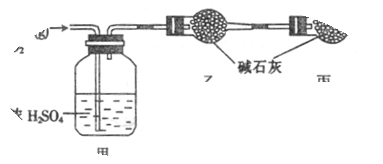
����ʵ��������գ�
��1����ȼ�Ϸ�����̼����ԭ�ӵ���Ŀ��Ϊ���� ������
��2����֪��ȼ�Ϸ��ӵ�ʽ��Ϊ46����ÿ�������к���1����ԭ�ӣ��������ʽΪ

| | ʵ��ǰ | ʵ��� |
| ������ / g | 101.1 | 103.8 |
| �ҵ����� / g | 82.0 | 86.4 |
��1����ȼ�Ϸ�����̼����ԭ�ӵ���Ŀ��Ϊ���� ������
��2����֪��ȼ�Ϸ��ӵ�ʽ��Ϊ46����ÿ�������к���1����ԭ�ӣ��������ʽΪ
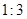 ��C2H6O
��C2H6O�����������1������֪��Ũ���������������ˮ��������Ϊ�������ɵ�����ȫ�������գ��ʼ�װ�����ӵ�����ΪH2O����������m(H2O)=103.8-101.1=2.7g��H������=2.7g*��2/18��=0.3g������ʯ�ҿ�����������CO2������װ�����ӵ�����ΪCO2����������m(CO2)=86.4-82.0=4.4g��C������=4.4g*��12/44��=1.2g����̼����ԭ�ӵ���Ŀ��Ϊ��1.2/12��:(0.3/1)=1:3��
��2����÷��Ӻ���n��̼ԭ�ӣ�����ԭ��Ϊ3n������������Ϊ12n+3n+16=46����n=2�����Ա����÷�����2��̼ԭ�ӣ�6����ԭ�Ӻ�һ����ԭ�ӣ��ʸ�����ΪC2H6O��
����������ͨ�������жϸ����ռ������յ��������������C��H�������������е��Ѷȵ��⣬Ҫ��ѧ�����ճ����ĸ���������ռ���

��ϰ��ϵ�д�
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д�
�����Ŀ

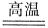 2Fe+3CO
2Fe+3CO ���˷�Ӧ�����ڹ�ҵ��ұ������������Ӧ�У���Ϊ��������������________���ѧʽ�����ڸ÷�Ӧ�У���������1 mol
���˷�Ӧ�����ڹ�ҵ��ұ������������Ӧ�У���Ϊ��������������________���ѧʽ�����ڸ÷�Ӧ�У���������1 mol  ����ת����_____mol���ӣ����ɵ�CO
����ת����_____mol���ӣ����ɵ�CO