��Ŀ����
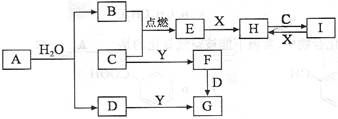
A��B��C��D��E��F�����ֶ�����Ԫ�أ����ǵ�ԭ�������������� A��D��C��FΪͬ����Ԫ�أ�A�ĵ�������������壻 BԪ������������ˮ����������̬�⻯�ﷴӦ�����Σ� EԪ���ǵؿ��к������Ľ���Ԫ�ء�FԪ��ԭ��M���ϵ�������L����2�����ӡ� ������������
��1��EԪ��λ�����ڱ��ĵ� ���� �塣
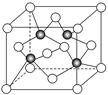
��2��F�������ӵĽṹʾ��ͼ ��
��3��ͭ��BԪ������������ˮ�����ϡ��Һ��Ӧ�����ӷ���ʽΪ ��
��4��E����������Һ�е���BԪ����̬�⻯���ˮ��Һ��ֱ�����������ӷ���ʽΪ ��
��1��EԪ��λ�����ڱ��ĵ� ���� �塣
��2��F�������ӵĽṹʾ��ͼ ��
��3��ͭ��BԪ������������ˮ�����ϡ��Һ��Ӧ�����ӷ���ʽΪ ��
��4��E����������Һ�е���BԪ����̬�⻯���ˮ��Һ��ֱ�����������ӷ���ʽΪ ��
��8�֣���1��������A ��2�֣� ��2�� 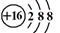 ��2�֣�
��2�֣�
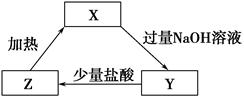
��3��3Cu��8H��+2NO3��=3Cu2����2NO����4H2O ��2�֣� ��4��Al3+��3NH3��H2O= 3NH4+��Al(OH)3�� ��2�֣�
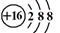 ��2�֣�
��2�֣���3��3Cu��8H��+2NO3��=3Cu2����2NO����4H2O ��2�֣� ��4��Al3+��3NH3��H2O= 3NH4+��Al(OH)3�� ��2�֣�
���������A�ĵ�������������壬����A����Ԫ�ء�BԪ������������ˮ����������̬�⻯�ﷴӦ�����Σ������������ǵ�Ԫ�أ���B�ǵ�Ԫ�ء�EԪ���ǵؿ��к������Ľ���Ԫ�أ�����E��AlԪ�ء�FԪ��ԭ��M���ϵ�������L����2�����ӣ���F��ԭ��������16����M��SԪ�ء�A��D��C��FΪͬ����Ԫ�أ����Ը���A��B��C��D��E��F��ԭ���������������֪��C����Ԫ�أ�D����Ԫ�ء�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ������֪ʶ�Թ��̺�ѵ���������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԡ�����������Ҫ���ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
�����Ŀ