��Ŀ����
���������ȷֽ�IJ����������ε�����Լ����ȵ��¶ȵ������йأ�
(1)һ����������������ȷֽ�Ļ�ѧ��Ӧ����ʽΪ��
NH4NO3��HNO3��N2��H2O(δ��ƽ)
��Ӧ�����ɵ�����������__________��
�ڷ�Ӧ�б������뱻��ԭ�ĵ�ԭ����֮��Ϊ__________��
��Ӧ��������0.2 mol��N2���壬��ת�Ƶ��ӵ����ʵ�����________mol��
(2)ij���������ȷֽ�IJ���Ϊ������Ԫ�صĹ������ʺ�NO2��O2���壬������NO2��O2�����ʵ���֮��Ϊ8��1�������Ԫ�صļ�̬�ڷ�Ӧ������________(����ߡ��������͡��������䡱)��
�𰸣�
������
������
(ÿ��2�֣���8��)(1)N2��5��3��0.75��(2)���ߣ�

��ϰ��ϵ�д�
�����Ŀ
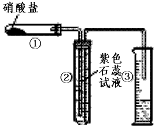
 2Ag2O��4NO2����O2���� (��) 2AgNO3
2Ag2O��4NO2����O2���� (��) 2AgNO3 2Ag��2NO2����O2����
2Ag��2NO2����O2����