��Ŀ����
��11�֣�����ij���������λ�ѧʽΪM��NO3��2��Ϊ��̽�����ȷֽ����,ij��ѧС�鿪չ̽����ѧϰ��
[��������]���������Բ�ͬ���������ηֽ���ﲻͬ���磬2KNO3 2KNO2+O2����
2KNO2+O2����
2Cu��NO3��2 2CuO+2NO2��+O2����4AgNO3
2CuO+2NO2��+O2����4AgNO3 4Ag+4NO2��+O2��
4Ag+4NO2��+O2��
[�������]����1 M��NO3��2 M��NO2��2+O2��
M��NO2��2+O2��
����2 2 M��NO3��2 2MO+4NO2��+O2��
2MO+4NO2��+O2��
����3 ��
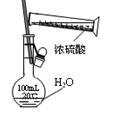
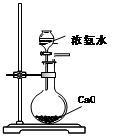
[���ʵ��]Ϊ��̽���������룬�������ʵ��װ�ã�
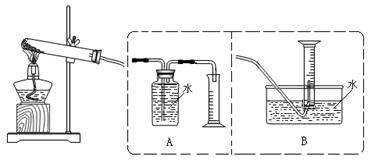
ʵ�鲽�裺�������������ڼ��װ�������ԣ���ȡһ������Ʒװ�ڴ��Թܣ����������Ӻ��������ܼ��ȣ��ݡ���
��1��д������3�Ļ�ѧ����ʽ�� ��
��2���ⶨ�������Ӧѡ�� ����A��B������ѡ��һ��װ�õ������� ��
��3��ʵ���ռ���һ�������壬Ϊ�˼����ռ�����ijɷ֣������������ ��
��4����ʵ����ˮ���������̼�����Ʒ�ĩ����Һ�в����������壬˵�� ��ȷ�������1������2�����3����
��5����С�����������ݣ���ʵ��ǰ��Ʒ����ΪWg���ռ����������ΪV L��
��ʵ��ǰ��Ʒ����ΪWg����ȫ�ֽ���ù����������Ϊm g��
����ѡ������һ�����ݣ�����M�����ԭ������Ϊ ��
[��������]���������Բ�ͬ���������ηֽ���ﲻͬ���磬2KNO3
 2KNO2+O2����
2KNO2+O2����2Cu��NO3��2
 2CuO+2NO2��+O2����4AgNO3
2CuO+2NO2��+O2����4AgNO3 4Ag+4NO2��+O2��
4Ag+4NO2��+O2��[�������]����1 M��NO3��2
 M��NO2��2+O2��
M��NO2��2+O2������2 2 M��NO3��2
 2MO+4NO2��+O2��
2MO+4NO2��+O2������3 ��
[���ʵ��]Ϊ��̽���������룬�������ʵ��װ�ã�

ʵ�鲽�裺�������������ڼ��װ�������ԣ���ȡһ������Ʒװ�ڴ��Թܣ����������Ӻ��������ܼ��ȣ��ݡ���
��1��д������3�Ļ�ѧ����ʽ�� ��
��2���ⶨ�������Ӧѡ�� ����A��B������ѡ��һ��װ�õ������� ��
��3��ʵ���ռ���һ�������壬Ϊ�˼����ռ�����ijɷ֣������������ ��
��4����ʵ����ˮ���������̼�����Ʒ�ĩ����Һ�в����������壬˵�� ��ȷ�������1������2�����3����
��5����С�����������ݣ���ʵ��ǰ��Ʒ����ΪWg���ռ����������ΪV L��
��ʵ��ǰ��Ʒ����ΪWg����ȫ�ֽ���ù����������Ϊm g��
����ѡ������һ�����ݣ�����M�����ԭ������Ϊ ��
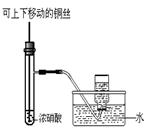
��1��M��NO3��2 M+2NO2��+O2����3�֣���2��B ��2�֣�װ��A��Ӧ�����ܽ���������������ˮ��������ˮ�ܿ�Ӧ������Ͳ���²����Ա����ǰ��ƽҺ�棨2�֣�
M+2NO2��+O2����3�֣���2��B ��2�֣�װ��A��Ӧ�����ܽ���������������ˮ��������ˮ�ܿ�Ӧ������Ͳ���²����Ա����ǰ��ƽҺ�棨2�֣�
��3���ò���Ƭ��ˮ����Ǻ���Ͳ�ڣ���ʳָ��������Ƭ������Ͳ��ˮ�����ó�����������ʵ��̨�ϣ��ƿ�����Ƭ���ô�����ľ���ӽ���Ͳ�ڣ���ľ��ȼ�ո�����˵���ռ�����������������2�֣���4������3��2�֣���5��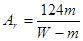 ��3�֣�
��3�֣�
 M+2NO2��+O2����3�֣���2��B ��2�֣�װ��A��Ӧ�����ܽ���������������ˮ��������ˮ�ܿ�Ӧ������Ͳ���²����Ա����ǰ��ƽҺ�棨2�֣�
M+2NO2��+O2����3�֣���2��B ��2�֣�װ��A��Ӧ�����ܽ���������������ˮ��������ˮ�ܿ�Ӧ������Ͳ���²����Ա����ǰ��ƽҺ�棨2�֣���3���ò���Ƭ��ˮ����Ǻ���Ͳ�ڣ���ʳָ��������Ƭ������Ͳ��ˮ�����ó�����������ʵ��̨�ϣ��ƿ�����Ƭ���ô�����ľ���ӽ���Ͳ�ڣ���ľ��ȼ�ո�����˵���ռ�����������������2�֣���4������3��2�֣���5��
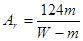 ��3�֣�
��3�֣���1��������֪��3����Ӧ��֪������3�Ļ�ѧ����ʽΪM��NO3��2 M+2NO2��+O2����
M+2NO2��+O2����
��2������װ��A�г����ܲ��뵽��Һ�У�����������ˮ��������ε�ˮ�ܿ�Ӧ������Ͳ���²����Ա����ǰ��ƽҺ�棬����Ӧ��ѡ��Bװ�á�
��3���������������ô����ǵ�ľ�����м��飬���ò���Ƭ��ˮ����Ǻ���Ͳ�ڣ���ʳָ��������Ƭ������Ͳ��ˮ�����ó�����������ʵ��̨�ϣ��ƿ�����Ƭ���ô�����ľ���ӽ���Ͳ�ڣ���ľ��ȼ�ո�����˵���ռ�����������������
��4������̼�������ܲ������ݣ�˵����Һ�������ԣ������ռ������л����������ᣬ���Բ�������ȷ�ġ�
��5�����ڷ�Ӧ�ķ�ʽ��ȷ�������Ԣ���������ʹ�ã������յĹ���һ����M������ѡ��������ݡ�����Mԭ���غ��֪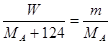 �����MA��
�����MA��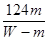 ��
��
 M+2NO2��+O2����
M+2NO2��+O2������2������װ��A�г����ܲ��뵽��Һ�У�����������ˮ��������ε�ˮ�ܿ�Ӧ������Ͳ���²����Ա����ǰ��ƽҺ�棬����Ӧ��ѡ��Bװ�á�
��3���������������ô����ǵ�ľ�����м��飬���ò���Ƭ��ˮ����Ǻ���Ͳ�ڣ���ʳָ��������Ƭ������Ͳ��ˮ�����ó�����������ʵ��̨�ϣ��ƿ�����Ƭ���ô�����ľ���ӽ���Ͳ�ڣ���ľ��ȼ�ո�����˵���ռ�����������������
��4������̼�������ܲ������ݣ�˵����Һ�������ԣ������ռ������л����������ᣬ���Բ�������ȷ�ġ�
��5�����ڷ�Ӧ�ķ�ʽ��ȷ�������Ԣ���������ʹ�ã������յĹ���һ����M������ѡ��������ݡ�����Mԭ���غ��֪
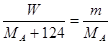 �����MA��
�����MA��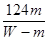 ��
��
��ϰ��ϵ�д�
�����Ŀ
 ����������
���������� ����������
���������� ����������
���������� ����������
����������