��Ŀ����
(10��) ��0.1 mol��þ�������������100 mL 2mol/LH2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����ش�
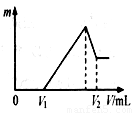
�����ڵμ�NaOH��Һ�Ĺ����У���������m�����NaOH��Һ����
��V�仯����ͼ��ʾ����V1��140mLʱ,�������ĩ��
n(Mg)������ mol��V2������������mL��
�����ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�����
��NaOH��Һ�����V(NaOH)��������mL��
�����������Ϊ0.1 mol������Mg�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���480 mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ�ǣ���������������������
(4)���μ�NaOH��Һ��V2mlʱ,ֹͣ�μ�NaOH��Һ����ʼ����Һ��ͨ������CO2,��д��������Ӧ�����ӷ���ʽ��������������������������������������������-����������
��1��0.04mol;460ml (2)400ml (3) 1> a�R0.2
(4)CO2+2H2O+AlO2-=Al(OH)3 +HCO3-
����:

��ϰ��ϵ�д�
 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
�����Ŀ

 CH3COO��+H+ ��H��0��
CH3COO��+H+ ��H��0�� CH3COO��+H+ ��H��0��
CH3COO��+H+ ��H��0��