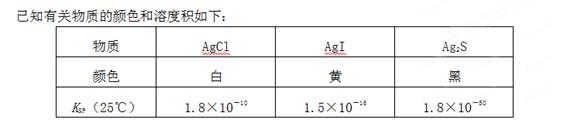
��Ŀ����
25�棬��0.01molCH3COONa��0.002molHCl����ˮ���γ�1L�����Һ���ش��������⣺
��1������Һ�д���������ƽ����ϵ���õ��뷽��ʽ�����ӷ���ʽ��ʾ��
��______����______����______��
��2����Һ�й���______�ֲ�ͬ�����ӣ�ָ���Ӻ����ӣ���
��3������Щ�����У�Ũ��Ϊ0.01mol?L-1����______��Ũ��Ϊ0.002mol?L-1����______��
��4��______��______�����������ʵ���֮�͵���0.01mol��
��1������Һ�д���������ƽ����ϵ���õ��뷽��ʽ�����ӷ���ʽ��ʾ��
��______����______����______��
��2����Һ�й���______�ֲ�ͬ�����ӣ�ָ���Ӻ����ӣ���
��3������Щ�����У�Ũ��Ϊ0.01mol?L-1����______��Ũ��Ϊ0.002mol?L-1����______��
��4��______��______�����������ʵ���֮�͵���0.01mol��
��1������ˮ�ĵ���ƽ�⡢����ĵ���ƽ�⡢��������ӵ�ˮ��ƽ�⣬�ֱ�ΪH2O?H++OH-��CH3COOH?CH3COO-+H+��CH3COO-+H2O?CH3COOH+OH-��
�ʴ�Ϊ��H2O?H++OH-��CH3COOH?CH3COO-+H+��CH3COO-+H2O?CH3COOH+OH-��
��2����ˮ��������ӡ������ӡ����������ӡ���������ӡ������ӡ������ӣ���7�������ʴ�Ϊ��7����
��3����0.01molCH3COONa����Ũ��Ϊ0.01mol?L-1����Na+����0.002molHCl����Ũ��Ϊ0.002mol?L-1����Cl-���ʴ�Ϊ��Na+��Cl-����
��4����0.01molCH3COONa����CH3COOH��CH3COO-��֮��Ϊ0.01mol���ʴ�Ϊ��CH3COOH��CH3COO-��
�ʴ�Ϊ��H2O?H++OH-��CH3COOH?CH3COO-+H+��CH3COO-+H2O?CH3COOH+OH-��
��2����ˮ��������ӡ������ӡ����������ӡ���������ӡ������ӡ������ӣ���7�������ʴ�Ϊ��7����
��3����0.01molCH3COONa����Ũ��Ϊ0.01mol?L-1����Na+����0.002molHCl����Ũ��Ϊ0.002mol?L-1����Cl-���ʴ�Ϊ��Na+��Cl-����
��4����0.01molCH3COONa����CH3COOH��CH3COO-��֮��Ϊ0.01mol���ʴ�Ϊ��CH3COOH��CH3COO-��

��ϰ��ϵ�д�
 ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ
 ��2��һ���¶��µ����ܵ����AmBn��ˮ��Һ�дﵽ�����ܽ�ƽ��ʱ����ƽ�ⳣ��
��2��һ���¶��µ����ܵ����AmBn��ˮ��Һ�дﵽ�����ܽ�ƽ��ʱ����ƽ�ⳣ��