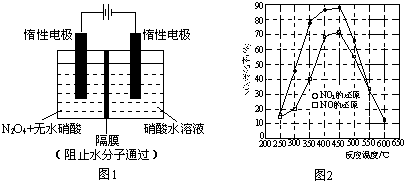
��Ŀ����
SCR����ѡ���Դ���ԭ��������һ����NH3��Ϊ��ԭ������������NOx�ֽ������N2��H2O�ĸɷ�������������Ӧԭ��Ϊ����6NO+4NH3��5N2+6H2O����6NO2+8NH3��7N2+12H2O����NO+NO2+2NH3��2N2+3H2O
����˵����ȷ����
A��NOx��Ҫ����������β�����ŷţ�����������ЧӦ����Ҫ����֮һ
B��N2�Цм���Ҽ�֮��Ϊ1:2
C����Ӧ����ÿ����22.4LN2��ת�Ƶ�����1.5NA
D��NH3�ķе��PH3�ķе��
D
��������
���������A��NOx��Ҫ����������β�����ŷţ�������������ЧӦ��������⻯ѧ��������Ҫ���壬����B��N2�Цм���Ҽ�֮��Ϊ2:1������C��û��ע����״����22.4L N2 �����ʵ�����ȷ��������D��NH3���Ӽ��γ��������ʹNH3�ķе����PH3����ȷ��
���㣺���⿼���������Ⱦ����ѧ�������͡���������ʵ�����

| A��NOX��Ҫ����������β�����ŷţ�����������ЧӦ����Ҫ����֮һ | B��N2�Ц�����O��֮��Ϊ1��2 | C����Ӧ����ÿ����22.4LN2��ת�Ƶ�����1.5NA | D��NH3�ķе��PH3�ķе�� |
(12��)���ŵ���������Ⱦ���������أ��ҹ����ڡ�ʮ���塱�ڼ�Ӵ�Ե��������ŷŵĿ������ȡ�Ŀǰ����������������Ⱦ�ж��ַ�����
��1���û���̿��ԭ��������������йط�ӦΪ��C��g��+2NO(g) N2(g)+CO2(g) ��H.ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ�̲�ø����ʵ�Ũ�����£�
N2(g)+CO2(g) ��H.ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ�̲�ø����ʵ�Ũ�����£�
| Ũ��/mol?L-1 ʱ��/min | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.040 | 0.030 | 0.030 |
| 30 | 0.040 | 0.030 | 0.030 |
| 40 | 0.032 | 0.034 | 0.017 |
| 50 | 0.032 | 0.034 | 0.017 |
��30min�ı�ijһ����������һ��ʱ���Ӧ���´ﵽƽ�⣬��ı������������
����30min�������¶���T2�棬�ﵽƽ��ʱ�������е�NO��N2��CO2��Ũ��֮��Ϊ5:3:3����÷�Ӧ�ġ�H �����������������=����0
��2����CH4����ԭ��������������������������Ⱦ����֪��
��CH4��g��+4NO2(g)=4NO(g)+CO2(g)+2H20(g) ��H=-574kJ.mol-1
��CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=-1160kJ.mol-1
��H2O(g)=H2O(l) ��H=-44.0kJ.mol-1
д��CH4��NO2��Ӧ����N2(g) ��CO2(g)�� H2O(l)���Ȼ�ѧ����ʽ��
��3��ѡ���Դ���ԭ��SCR������������ĿǰӦ����㡢����Ч��������������֮һ���ü����漰���·�Ӧ��4NO(g)+4NH3(g)+ O2(g)=��4N2(g)+ 6H2O(g),�ں��ݵ��ܱ������У������й�˵������ȷ���� ������ĸ��
A�������������䣬ʹ�ø�Ч������������NO��ת��������
B����λʱ��������NH3��NO�����ʵ���֮��Ϊ1:1ʱ����Ӧ�ﵽƽ��
C��������������ʱ�����¶ȣ���Ӧ��ƽ�ⳣ����С
D����Ӧ�ﵽƽ������������г�������ķ�Ӧ��ٴδﵽƽ��ʱ��NO��ת���ʼ�С
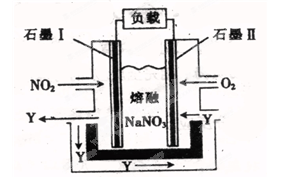
��4����NO2 ��O2������NaNO3��ɵ�ȼ�ϵ��װ����ͼ��ʾ����ʹ�ù�����ʯī��缫��Ӧ����һ��������Y���õ缫�ĵ缫��Ӧ�ɱ�ʾΪ
(12��)���ŵ���������Ⱦ���������أ��ҹ����ڡ�ʮ���塱�ڼ�Ӵ�Ե��������ŷŵĿ������ȡ�Ŀǰ����������������Ⱦ�ж��ַ�����
��1���û���̿��ԭ��������������йط�ӦΪ��C��g��+2NO(g) N2(g)+CO2(g)
��H.ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ�̲�ø����ʵ�Ũ�����£�
N2(g)+CO2(g)
��H.ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ�̲�ø����ʵ�Ũ�����£�
|
Ũ��/mol•L-1 ʱ��/min |
NO |
N2 |
CO2 |
|
0 |
0.100 |
0 |
0 |
|
10 |
0.058 |
0.021 |
0.021 |
|
20 |
0.040 |
0.030 |
0.030 |
|
30 |
0.040 |
0.030 |
0.030 |
|
40 |
0.032 |
0.034 |
0.017 |
|
50 |
0.032 |
0.034 |
0.017 |
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K= ��������λС����
��30min�ı�ijһ����������һ��ʱ���Ӧ���´ﵽƽ�⣬��ı������������
����30min�������¶���T2�棬�ﵽƽ��ʱ�������е�NO��N2��CO2��Ũ��֮��Ϊ5:3:3����÷�Ӧ�ġ�H �����������������=����0
��2����CH4����ԭ��������������������������Ⱦ����֪��
��CH4��g��+4NO2(g)=4NO(g)+CO2(g)+2H20(g) ��H=-574kJ.mol-1
��CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=-1160kJ.mol-1
��H2O(g)=H2O(l) ��H=-44.0kJ.mol-1
д��CH4��NO2��Ӧ����N2(g) ��CO2(g)�� H2O(l)���Ȼ�ѧ����ʽ��
��3��ѡ���Դ���ԭ��SCR������������ĿǰӦ����㡢����Ч��������������֮һ���ü����漰���·�Ӧ��4NO(g)+4NH3(g)+ O2(g)=��4N2(g)+ 6H2O(g),�ں��ݵ��ܱ������У������й�˵������ȷ���� ������ĸ��
A�������������䣬ʹ�ø�Ч������������NO��ת��������
B����λʱ��������NH3��NO�����ʵ���֮��Ϊ1:1ʱ����Ӧ�ﵽƽ��
C��������������ʱ�����¶ȣ���Ӧ��ƽ�ⳣ����С
D����Ӧ�ﵽƽ������������г�������ķ�Ӧ��ٴδﵽƽ��ʱ��NO��ת���ʼ�С
��4����NO2 ��O2������NaNO3��ɵ�ȼ�ϵ��װ����ͼ��ʾ����ʹ�ù�����ʯī��缫��Ӧ����һ��������Y���õ缫�ĵ缫��Ӧ�ɱ�ʾΪ