��Ŀ����
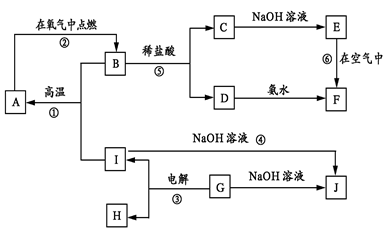
��12�֣���ҵ����������(��Ҫ�ɷ�ΪAl2O3��Fe2O3��)��ȡAl2O3��ұ������ԭ�ϣ������ε�ⷨ��õĴ����к�һ�����Ľ����ƺ���������Щ���ʿɲ��ô�����������ȥ��������β��������������ڸֲĶ�����������������ͼ��ʾ��
(��֪��NaCl�۵�Ϊ801�棻AlCl3��181������)

��1������Һ��ͨ�����CO2��������Ӧ�����ӷ���ʽΪ ��
��2������ǰ������������������������ʯӢɰ����ֹ����ʱ���Ƿֱ����������û���Ӧ�����µ����ʣ���������������Ӧ�Ļ�ѧ����ʽΪ ��
��3����Cl2����ͨ�������еĴ������壬�����������ϸ�����ȥ�����ݵ���Ҫ�ɷֳ�Cl2�����______����̬����ճ���������ϣ�����������γɸ����������п϶�����________��
��4�����������У�������Ϊ�����������ε��Һ����Ԫ����Ҫ��AlCl4����ʽ���ڣ��������ĵ缫��ӦʽΪ_____________��
��5���ֲĶ�������ʴ���ܻ�����ǿ����ԭ����_____________��
(��֪��NaCl�۵�Ϊ801�棻AlCl3��181������)

��1������Һ��ͨ�����CO2��������Ӧ�����ӷ���ʽΪ ��
��2������ǰ������������������������ʯӢɰ����ֹ����ʱ���Ƿֱ����������û���Ӧ�����µ����ʣ���������������Ӧ�Ļ�ѧ����ʽΪ ��
��3����Cl2����ͨ�������еĴ������壬�����������ϸ�����ȥ�����ݵ���Ҫ�ɷֳ�Cl2�����______����̬����ճ���������ϣ�����������γɸ����������п϶�����________��
��4�����������У�������Ϊ�����������ε��Һ����Ԫ����Ҫ��AlCl4����ʽ���ڣ��������ĵ缫��ӦʽΪ_____________��
��5���ֲĶ�������ʴ���ܻ�����ǿ����ԭ����_____________��
��12�֣�
��1�� AlO2�� ��2H2O ��CO2��Al(OH)3����HCO3����2�֣�
��2��Fe2O3��2Al Al2O3��2Fe��2�֣�
Al2O3��2Fe��2�֣�
��3��HCl��AlCl3����2�֣� NaCl��2�֣�
��4��Al-3e-��4Cl-= AlCl4-��2�֣�
��5�������γɵ�����������Ĥ�ܷ�ֹ�ֲĸ�ʴ�������ܵ�������Ĥ�������еĵ������Һ���ڲ�������� ��2�֣�
��1�� AlO2�� ��2H2O ��CO2��Al(OH)3����HCO3����2�֣�
��2��Fe2O3��2Al
 Al2O3��2Fe��2�֣�
Al2O3��2Fe��2�֣���3��HCl��AlCl3����2�֣� NaCl��2�֣�
��4��Al-3e-��4Cl-= AlCl4-��2�֣�
��5�������γɵ�����������Ĥ�ܷ�ֹ�ֲĸ�ʴ�������ܵ�������Ĥ�������еĵ������Һ���ڲ�������� ��2�֣�
�����������1����Һ�к���AlO2�� ��ͨ��CO2������Al(OH)3���������ӷ���ʽΪ��AlO2�� ��2H2O ��CO2��Al(OH)3����HCO3��
��2�����ڸ��������£���Fe2O3��ԭΪFe����ѧ����ʽΪ��Fe2O3��2Al
 Al2O3��2Fe
Al2O3��2Fe��3����Ϊ�����к���������������Cl2��Ӧ����AlCl3��H2��AlCl3��181���������������ݵ���Ҫ�ɷֳ�Cl2�����HCl��AlCl3�������к���Na����Cl2��Ӧ����NaCl��NaCl�۵�Ϊ801�棬���Ը����п϶�����NaCl��
��4��Alʧ��������AlCl4�����缫����ʽΪ��Al-3e-��4Cl-= AlCl4-
��5��Al�ڿ�������O2��Ӧ����Al2O3�������γɵ�����������Ĥ���ܷ�ֹ�ֲĸ�ʴ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ