��Ŀ����
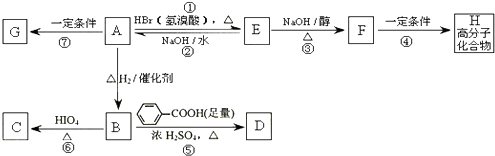
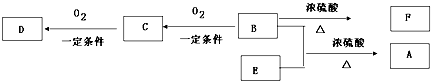
���㻯����A��B��Ϊͬ���칹�壬B�Ľṹ��ʽ�ǣ�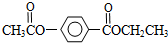 A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��
A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��
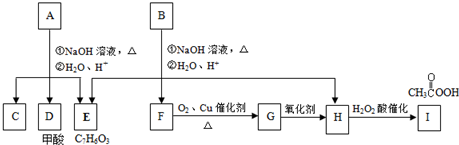
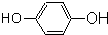
��1��д��E�Ľṹ��ʽE______��E�����������ŵ�������______��
��2��A��2�ֿ��ܵĽṹ��д����Ӧ�Ľṹ��ʽ______��______
��3��д��B��NaOH��Һ���ȵĻ�ѧ����ʽ______
��4��д��F��H�ڼ��Ⱥ�ŨH2SO4�������·�����Ӧ�Ļ�ѧ����ʽ______����Ӧ������______��
��5����B��C��D��F��G��I�������У���Ϊͬϵ�����______��
 A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��
A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��
��1��д��E�Ľṹ��ʽE______��E�����������ŵ�������______��
��2��A��2�ֿ��ܵĽṹ��д����Ӧ�Ľṹ��ʽ______��______
��3��д��B��NaOH��Һ���ȵĻ�ѧ����ʽ______
��4��д��F��H�ڼ��Ⱥ�ŨH2SO4�������·�����Ӧ�Ļ�ѧ����ʽ______����Ӧ������______��
��5����B��C��D��F��G��I�������У���Ϊͬϵ�����______��
B���������Ƶ�ˮ��Һ������ˮ�⡢�ữ�õ�CH3CH2OH��CH3COOH��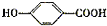 �����E�ķ���ʽ��֪��EΪ
�����E�ķ���ʽ��֪��EΪ ��F����������G��G��������H����FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH��H�ڹ��������ữ�����������ɹ������ᣮ
��F����������G��G��������H����FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH��H�ڹ��������ữ�����������ɹ������ᣮ
A���������Ƶ�ˮ��Һ������ˮ�⡢�ữ�õ�C��HCOOH�� ����CΪCH3CH2CH2OH��CH3CH��OH��CH3����AΪ
����CΪCH3CH2CH2OH��CH3CH��OH��CH3����AΪ ��
�� ��
��
��1��������������֪��E�Ľṹ��ʽΪ ��E�й������������Ȼ����ǻ����ʴ�Ϊ��
��E�й������������Ȼ����ǻ����ʴ�Ϊ�� ���Ȼ����ǻ���
���Ȼ����ǻ���
��2��������������֪��AΪ ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��
��
��3��B��NaOH��Һ����ˮ�ⷴӦ���䷴Ӧ�Ļ�ѧ��Ӧ����ʽ +3NaOH��CH3COONa+
+3NaOH��CH3COONa+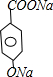 +CH3CH2OH+H2O��
+CH3CH2OH+H2O��
�ʴ�Ϊ�� +3NaOH��CH3COONa+
+3NaOH��CH3COONa+ +CH3CH2OH+H2O��
+CH3CH2OH+H2O��
��4��F��H�ڼ��Ⱥ�ŨH2SO4�������·���������Ӧ����������������Ӧ��ѧ����ʽΪCH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��������Ӧ��
��5��ͬϵ���Ǿ�����ͬ�Ĺ����š�����ͬһ�����ʡ�ͨʽ��ͬ�ҷ�����������һ�������ɸ�-CH2-���ŵ��л���֮��Ļ��ƣ���B��C��D��F��G��I�������У�ֻ��C��F������������C��F��Ϊͬϵ�
�ʴ�Ϊ��C��F��
 �����E�ķ���ʽ��֪��EΪ
�����E�ķ���ʽ��֪��EΪ ��F����������G��G��������H����FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH��H�ڹ��������ữ�����������ɹ������ᣮ
��F����������G��G��������H����FΪCH3CH2OH��GΪCH3CHO��HΪCH3COOH��H�ڹ��������ữ�����������ɹ������ᣮA���������Ƶ�ˮ��Һ������ˮ�⡢�ữ�õ�C��HCOOH��
 ����CΪCH3CH2CH2OH��CH3CH��OH��CH3����AΪ
����CΪCH3CH2CH2OH��CH3CH��OH��CH3����AΪ ��
�� ��
����1��������������֪��E�Ľṹ��ʽΪ
 ��E�й������������Ȼ����ǻ����ʴ�Ϊ��
��E�й������������Ȼ����ǻ����ʴ�Ϊ�� ���Ȼ����ǻ���
���Ȼ����ǻ�����2��������������֪��AΪ
 ��
�� ��
���ʴ�Ϊ��
 ��
�� ��
����3��B��NaOH��Һ����ˮ�ⷴӦ���䷴Ӧ�Ļ�ѧ��Ӧ����ʽ
 +3NaOH��CH3COONa+
+3NaOH��CH3COONa+ +CH3CH2OH+H2O��
+CH3CH2OH+H2O���ʴ�Ϊ��
 +3NaOH��CH3COONa+
+3NaOH��CH3COONa+ +CH3CH2OH+H2O��
+CH3CH2OH+H2O����4��F��H�ڼ��Ⱥ�ŨH2SO4�������·���������Ӧ����������������Ӧ��ѧ����ʽΪCH3CH2OH+CH3COOH
| ŨH2SO4 |
| �� |
�ʴ�Ϊ��CH3CH2OH+CH3COOH
| ŨH2SO4 |
| �� |
��5��ͬϵ���Ǿ�����ͬ�Ĺ����š�����ͬһ�����ʡ�ͨʽ��ͬ�ҷ�����������һ�������ɸ�-CH2-���ŵ��л���֮��Ļ��ƣ���B��C��D��F��G��I�������У�ֻ��C��F������������C��F��Ϊͬϵ�
�ʴ�Ϊ��C��F��

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
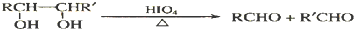
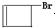 �ϳ�OHC��CH2��2CHO���÷�Ӧ����ͼ��ʾ��ע����Ӧ���Ӧ���������Լ�����ѡ������Ӧ����ͼʾ�������Ҵ��ϳɾ���ϩ����
�ϳ�OHC��CH2��2CHO���÷�Ӧ����ͼ��ʾ��ע����Ӧ���Ӧ���������Լ�����ѡ������Ӧ����ͼʾ�������Ҵ��ϳɾ���ϩ����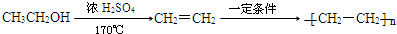

 ______��
______��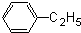 _______��
_______��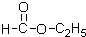 ______��
______��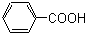 ______��
______��