��Ŀ����
��һ�ֿ������ϵĻ�����A��A��ֻ��C��H��O����Ԫ�أ�ͨ��������֪A�к���̼��˫����������C��H��Oԭ�Ӹ�����Ϊ2��4��1����Է�������Ϊ88����֪��
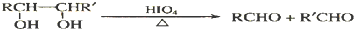
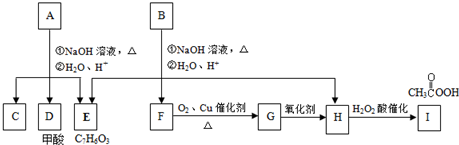
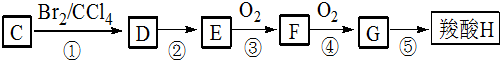
��A��صķ�Ӧ���£�
��1��A�ķ���ʽΪ______������7����Ӧ��������ȥ��Ӧ����______������ţ���
��2��д��B��D��Ӧ�Ļ�ѧ����ʽ��______��
��3��C�Ľṹ��ʽΪ______��F�Ľṹ��ʽΪ______��
��4��A�ڿ����г�ʱ����ã���ת��Ϊ��Է�������Ϊ86�Ļ�����G��G�ж���ͬ���칹�壬���з�����������������ͬ���칹�干��______�֣�
����״�ṹ�ڲ���̼̼˫����̼̼����
��5������ƺ���������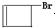 �ϳ�OHC��CH2��2CHO���÷�Ӧ����ͼ��ʾ��ע����Ӧ���Ӧ���������Լ�����ѡ������Ӧ����ͼʾ�������Ҵ��ϳɾ���ϩ����
�ϳ�OHC��CH2��2CHO���÷�Ӧ����ͼ��ʾ��ע����Ӧ���Ӧ���������Լ�����ѡ������Ӧ����ͼʾ�������Ҵ��ϳɾ���ϩ����
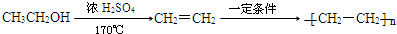
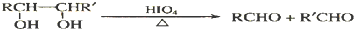
��A��صķ�Ӧ���£�

��1��A�ķ���ʽΪ______������7����Ӧ��������ȥ��Ӧ����______������ţ���
��2��д��B��D��Ӧ�Ļ�ѧ����ʽ��______��
��3��C�Ľṹ��ʽΪ______��F�Ľṹ��ʽΪ______��
��4��A�ڿ����г�ʱ����ã���ת��Ϊ��Է�������Ϊ86�Ļ�����G��G�ж���ͬ���칹�壬���з�����������������ͬ���칹�干��______�֣�
����״�ṹ�ڲ���̼̼˫����̼̼����
��5������ƺ���������
 �ϳ�OHC��CH2��2CHO���÷�Ӧ����ͼ��ʾ��ע����Ӧ���Ӧ���������Լ�����ѡ������Ӧ����ͼʾ�������Ҵ��ϳɾ���ϩ����
�ϳ�OHC��CH2��2CHO���÷�Ӧ����ͼ��ʾ��ע����Ӧ���Ӧ���������Լ�����ѡ������Ӧ����ͼʾ�������Ҵ��ϳɾ���ϩ����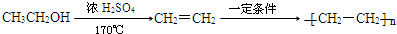
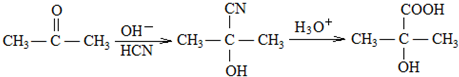
A������C��H��Oԭ�Ӹ�����Ϊ2��4��1�������ʽΪ��C2H4O��n������Է�������Ϊ88����44n=2�����n=2����A�ķ���ʽΪC4H8O2�������Ͷ�Ϊ
=1��A�к���̼��˫�������A��E���ת����֪��A�к��д��ǻ�-OH����Bת��ΪC�ķ�Ӧ������֪����Ӧ��Ϣ�еķ�Ӧ��B�к���2�����ڵ�-OH����A���еĹ�����Ϊ-OH��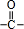 ��Bת��ΪC��������ֻ��һ�֣�B����Ϊ�Գƽṹ����A�Ľṹ��ʽΪ
��Bת��ΪC��������ֻ��һ�֣�B����Ϊ�Գƽṹ����A�Ľṹ��ʽΪ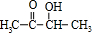 ����BΪ
����BΪ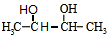 ��CΪCH3CHO��EΪ
��CΪCH3CHO��EΪ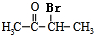 ��B�������ı����ᷢ��������Ӧ����D��DΪ
��B�������ı����ᷢ��������Ӧ����D��DΪ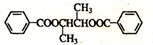 ��E���������ƴ���Һ�����������·�����ȥ��Ӧ����F��FΪ
��E���������ƴ���Һ�����������·�����ȥ��Ӧ����F��FΪ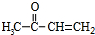 ��F�����Ӿ۷�Ӧ���ɸ߷��ӻ�����H��HΪ
��F�����Ӿ۷�Ӧ���ɸ߷��ӻ�����H��HΪ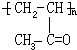 ��A��ת��Ϊ��Է�������Ϊ86�Ļ�����G����A��G����Է���������֪��Ӧ��A��-OH����Ϊ
��A��ת��Ϊ��Է�������Ϊ86�Ļ�����G����A��G����Է���������֪��Ӧ��A��-OH����Ϊ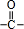 ����GΪ
����GΪ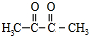 ��
��
��1��������������֪��A�ķ���ʽΪC4H8O2������7����Ӧ�з�Ӧ�٢ڢ�����ȡ����Ӧ����Ӧ��������ȥ��Ӧ����Ӧ�ޢ�����������Ӧ����Ӧ�����ڼӾ۷�Ӧ��
�ʴ�Ϊ��C4H8O2���ۣ�
��2��B��D��Ӧ�Ļ�ѧ����ʽΪ��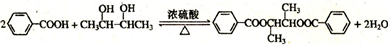 ��
��
�ʴ�Ϊ�� ��
��
��3����������������֪��C�Ľṹ��ʽΪCH3CHO��F�Ľṹ��ʽΪ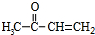 ��
��
�ʴ�Ϊ��CH3CHO��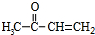 ��
��
��4��G��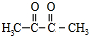 ����ͬ���칹������״�ṹ������̼̼˫����̼̼�������ʺ����ʻ���ȩ����ֻ����ȩ��������������ͬ���칹���У�CH3COCH2CHO��CH3CH2COCHO��OHCCH2CH2CHO��OHCC��CH3��CHO���ʴ�Ϊ��4��
����ͬ���칹������״�ṹ������̼̼˫����̼̼�������ʺ����ʻ���ȩ����ֻ����ȩ��������������ͬ���칹���У�CH3COCH2CHO��CH3CH2COCHO��OHCCH2CH2CHO��OHCC��CH3��CHO���ʴ�Ϊ��4��
��5���� �ϳ�OHC��CH2��2CHO�ķ�Ӧ����ͼΪ��
�ϳ�OHC��CH2��2CHO�ķ�Ӧ����ͼΪ��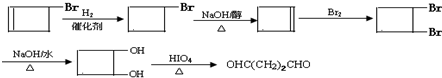 ��
��
�ʴ�Ϊ�� ��
��
| 2��4+2-8 |
| 2 |
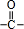 ��Bת��ΪC��������ֻ��һ�֣�B����Ϊ�Գƽṹ����A�Ľṹ��ʽΪ
��Bת��ΪC��������ֻ��һ�֣�B����Ϊ�Գƽṹ����A�Ľṹ��ʽΪ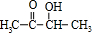 ����BΪ
����BΪ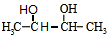 ��CΪCH3CHO��EΪ
��CΪCH3CHO��EΪ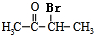 ��B�������ı����ᷢ��������Ӧ����D��DΪ
��B�������ı����ᷢ��������Ӧ����D��DΪ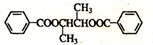 ��E���������ƴ���Һ�����������·�����ȥ��Ӧ����F��FΪ
��E���������ƴ���Һ�����������·�����ȥ��Ӧ����F��FΪ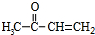 ��F�����Ӿ۷�Ӧ���ɸ߷��ӻ�����H��HΪ
��F�����Ӿ۷�Ӧ���ɸ߷��ӻ�����H��HΪ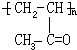 ��A��ת��Ϊ��Է�������Ϊ86�Ļ�����G����A��G����Է���������֪��Ӧ��A��-OH����Ϊ
��A��ת��Ϊ��Է�������Ϊ86�Ļ�����G����A��G����Է���������֪��Ӧ��A��-OH����Ϊ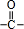 ����GΪ
����GΪ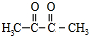 ��
����1��������������֪��A�ķ���ʽΪC4H8O2������7����Ӧ�з�Ӧ�٢ڢ�����ȡ����Ӧ����Ӧ��������ȥ��Ӧ����Ӧ�ޢ�����������Ӧ����Ӧ�����ڼӾ۷�Ӧ��
�ʴ�Ϊ��C4H8O2���ۣ�
��2��B��D��Ӧ�Ļ�ѧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����3����������������֪��C�Ľṹ��ʽΪCH3CHO��F�Ľṹ��ʽΪ
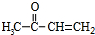 ��
���ʴ�Ϊ��CH3CHO��
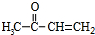 ��
����4��G��
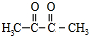 ����ͬ���칹������״�ṹ������̼̼˫����̼̼�������ʺ����ʻ���ȩ����ֻ����ȩ��������������ͬ���칹���У�CH3COCH2CHO��CH3CH2COCHO��OHCCH2CH2CHO��OHCC��CH3��CHO���ʴ�Ϊ��4��
����ͬ���칹������״�ṹ������̼̼˫����̼̼�������ʺ����ʻ���ȩ����ֻ����ȩ��������������ͬ���칹���У�CH3COCH2CHO��CH3CH2COCHO��OHCCH2CH2CHO��OHCC��CH3��CHO���ʴ�Ϊ��4����5����
 �ϳ�OHC��CH2��2CHO�ķ�Ӧ����ͼΪ��
�ϳ�OHC��CH2��2CHO�ķ�Ӧ����ͼΪ�� ��
���ʴ�Ϊ��
 ��
��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
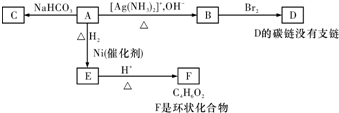
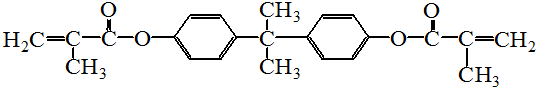
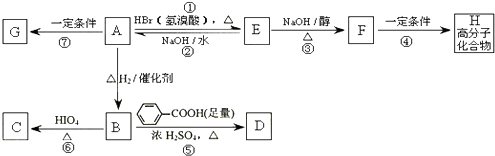
 A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��
A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ��ͼ��ʾ��