��Ŀ����
�����ǻ�ѧѧϰ���о��г��õ�˼ά�������������ƴ������
��Mg���ɵ������MgCl2��ȡ����AlҲ���ɵ������AlCl3��ȡ
�ھ������������ӣ����������ӣ��������������ӣ�Ҳ����������
��1mol/L��NaCl��Һÿ���к���1mol Na������1mol/L��FeCl3��Һÿ����Ҳ����1molFe3��
��ʹ��pH��ֽʱ���뱣�ָ����ʪ���pH��ֽ��õ�pHһ�������
| A���٢� | B���٢ۢ� | C���ڢۢ� | D���٢ڢۢ� |
D
���������������Mg���ɵ������MgCl2��ȡ����Alȴ���ɵ������Al2O3�ķ�����ȡ����ΪAlCl3���ɷ��ӹ��ɵ����ʣ����ܵ��ұ��Al�����ھ������������ӣ����������ӣ��������������������ӣ�ȴû�������ӡ�����1mol/L��NaCl��Һÿ���к���1mol Na������1mol/L��FeCl3��Һ������Fe3+ˮ�����ģ�����ÿ���к���Fe3��С��1mol������ʹ��pH��ֽʱ���뱣�ָ������ʪ���pH��ֽ��������Һ�õ�pHҲû��������
���㣺�������Ʒ��ڻ�ѧѧϰ���о���Ӧ��ʱӦ��ע��������֪ʶ��

 ������������ϵ�д�
������������ϵ�д����и������ʵľ����У���ѧ��������ͬ����������Ҳ��ͬ����
| A��SO2��Si | B��CO2��H2O |
| C��NaCl��HCl | D��CCl4��KCl |
���������۷е�ߵͱȽ���ȷ����
| A��SiO2<CO2 | B��CCl4<CF4 | C��NaCl<HCl | D��HF��HCl |
���ھ��������˵����ȷ����
| A��������ֻҪ�������Ӿ�һ���������� |
| B��������ֻҪ�������Ӿ�һ���������� |
| C��ԭ�Ӿ�����۵�һ���Ƚ�������ĸ� |
| D�����Ӿ�����۵�һ���Ƚ�������ĵ� |
��֪C3N4������бȽ��ʯ�����Ӳ�ȣ��ҹ��ɸþ��������ֻ�Ե�����ϡ����й���C3N4�����˵��������� (����)
| A���þ�������ԭ�Ӿ��壬�仯ѧ���Ƚ��ʯ�еĻ�ѧ�����ι� |
| B���þ�����ÿ��̼ԭ������4����ԭ�ӣ�ÿ����ԭ������3��̼ԭ�� |
| C���þ�����̼ԭ�Ӻ͵�ԭ�ӵ�����㶼����8���ӽṹ |
| D���þ�������ʯ���ƣ�����ԭ�Ӽ��ԷǼ��Լ��γɿռ���״�ṹ |
���ӱ������Ӽ�������������ʾ����������Һ��������ʾͼ������֡��й�����ʾԭ���������У���ȷ���� �� ����
| A��ʩ�ӵ糡ʱ��Һ�������ش�ֱ�ڵ糡�������� |
| B����ȥ�糡��Һ�����ӻָ���ԭ��״̬ |
| C��ʩ�ӵ糡��Һ�����ӻָ���ԭ��״̬ |
| D����ȥ�糡��Һ�������ص糡�������� |
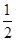 ��n��3��l��1��m��0��ms����
��n��3��l��1��m��0��ms����