��Ŀ����
Na2S2O3��5H2O���׳ƺ�������մ�������ҵ���õ�һ�ֶ�Ӱ����
��һ)��������;���Ʊ�������
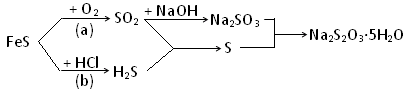
��֪��Ӧ��4FeS+7O2 2Fe2O3+4SO2��Na2SO3+S
2Fe2O3+4SO2��Na2SO3+S Na2S2O3
Na2S2O3
��1������ԭ��FeS�ڷ�Ӧ(a)��(b)�е����۷���ȣ�_________��
��2������88gFeS����NaOH��Һ����SO2��������Ϊ96%��������Ʊ���������Ϊ____�ˣ���ȷ��0��1�ˣ�
��������ҵ���Ƶõĺ��������п��ܺ����������������ƺ����������ʡ�Ϊ�˲ⶨij������Ʒ�ijɷ֣���ȡ����������ͬ�ĸ���Ʒ���ֱ������ͬŨ�ȵ�������Һ30mL����ַ�Ӧ����˳�������Һʹ���ɵ�SO2ȫ���ݳ���Na2S2O3+ H2SO4® Na2SO4+ SO2��+ S��+ H2O��������й�ʵ���������£���״������
��3����������������Һ�����ʵ���Ũ�ȡ�(д���������)
��4����������ʵ�����ݣ����жϸ���Ʒ__________������ĸ��
A������Na2SO3��Na2SO4 B������Na2SO3 ��Na2SO4
C����Na2SO3����Na2SO4 D����Na2SO3��Na2SO4
��5������30��16g����Ʒ��һ����������������Һ����ȡ������ۣ���������������______(aL)�ڲ�ͬȡֵ��Χʱ������SO2���___________(bL,��̬)��ֵ�������ú�a��b�Ĵ���ʽ��ʾ��
��һ)��������;���Ʊ�������
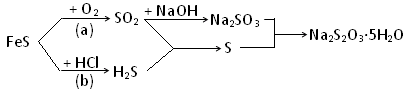
��֪��Ӧ��4FeS+7O2
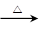 2Fe2O3+4SO2��Na2SO3+S
2Fe2O3+4SO2��Na2SO3+S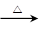 Na2S2O3
Na2S2O3��1������ԭ��FeS�ڷ�Ӧ(a)��(b)�е����۷���ȣ�_________��
��2������88gFeS����NaOH��Һ����SO2��������Ϊ96%��������Ʊ���������Ϊ____�ˣ���ȷ��0��1�ˣ�
��������ҵ���Ƶõĺ��������п��ܺ����������������ƺ����������ʡ�Ϊ�˲ⶨij������Ʒ�ijɷ֣���ȡ����������ͬ�ĸ���Ʒ���ֱ������ͬŨ�ȵ�������Һ30mL����ַ�Ӧ����˳�������Һʹ���ɵ�SO2ȫ���ݳ���Na2S2O3+ H2SO4® Na2SO4+ SO2��+ S��+ H2O��������й�ʵ���������£���״������
| | ��һ�� | �ڶ��� | ������ |
| ��Ʒ������/g | 7��54 | 15��08 | 35��00 |
| ������������/L | 0��672 | 1��344 | 2��688 |
| �������/g | 0��80 | 1��60 | 3��20 |
��3����������������Һ�����ʵ���Ũ�ȡ�(д���������)
��4����������ʵ�����ݣ����жϸ���Ʒ__________������ĸ��
A������Na2SO3��Na2SO4 B������Na2SO3 ��Na2SO4
C����Na2SO3����Na2SO4 D����Na2SO3��Na2SO4
��5������30��16g����Ʒ��һ����������������Һ����ȡ������ۣ���������������______(aL)�ڲ�ͬȡֵ��Χʱ������SO2���___________(bL,��̬)��ֵ�������ú�a��b�Ĵ���ʽ��ʾ��
��1��2:1
��2��121��5 g
��3��4mol/L
��4��D
��5��0<a<0��03Lʱ b=89��6a �� a��0��03Lʱ b=2��688
��2��121��5 g
��3��4mol/L
��4��D
��5��0<a<0��03Lʱ b=89��6a �� a��0��03Lʱ b=2��688
���������
��1��4FeS+7O2
 2Fe2O3+4SO2, FeS+2HCl=FeCl2+H2S����2H2S +SO2=3S+2H2O��SO2+2NaOH=Na2SO3+ H2O��Na2SO3+S
2Fe2O3+4SO2, FeS+2HCl=FeCl2+H2S����2H2S +SO2=3S+2H2O��SO2+2NaOH=Na2SO3+ H2O��Na2SO3+S Na2S2O3����ǡ����ȫ��Ӧ����Na2SO3��S=1:1�����ݷ���ʽ�������ƣ��ɵõ�FeS�ڷ�Ӧ(a)��(b)�е����۷����Ϊ2:1��
Na2S2O3����ǡ����ȫ��Ӧ����Na2SO3��S=1:1�����ݷ���ʽ�������ƣ��ɵõ�FeS�ڷ�Ӧ(a)��(b)�е����۷����Ϊ2:1����2��(1)n(FeS)=88g��88g/mol=1mol�����Ʊ�����������ࡣ����ݷ���ʽNa2SO3+S
 Na2S2O3��֪Na2SO3��S=1:1�����������Na2SO3�����ʵ���Ϊx������NaOH��Һ����SO2��������Ϊ96%����Ӧ���ĵ�SO2�����ʵ���Ϊx��0��96�������S��ȫ(1- x��0��96)��ȫת��ΪS���ʣ� 1- x��0��96=x�����x=0��4898�����Եõ���Na2S2O3��5H2O����Ϊ��0��4898mol��248g/mol=121��5 g��
Na2S2O3��֪Na2SO3��S=1:1�����������Na2SO3�����ʵ���Ϊx������NaOH��Һ����SO2��������Ϊ96%����Ӧ���ĵ�SO2�����ʵ���Ϊx��0��96�������S��ȫ(1- x��0��96)��ȫת��ΪS���ʣ� 1- x��0��96=x�����x=0��4898�����Եõ���Na2S2O3��5H2O����Ϊ��0��4898mol��248g/mol=121��5 g����3��ͨ���Ա��е����ݽ��з������ֵ���������������ȫ��Ӧ���йصķ�Ӧ����ʽΪNa2S2O3+ H2SO4= Na2SO4+ SO2��+ S��+ H2O��Na2SO3+ H2SO4= Na2SO4+ SO2��+H2O���ɼ��������ᷢ���Ǹ���Ӧ���й�ϵʽH2SO4��SO2��n(SO2)= 2��688L��22��4L/mol=0��12mol������n(H2SO4)= 0��12mol��c(H2SO4) =0��12mol��0��03L=4mol/L��
��4��n(S)=0��1mol,����ݷ���ʽNa2S2O3+ H2SO4= Na2SO4+ SO2��+ S��+ H2O��֪n(Na2S2O3)=0��1mol, m(Na2S2O3��5H2O)=24��8g;��������SO2Ҳ��0��1mol����Na2SO3������SO2Ϊ0��12mol-0��1mol=0��02mol������n(Na2SO3)=0��02mol��m(Na2SO3)=0��02mol��126g/mol=2��52g������m(Na2S2O3��5H2O)��m(Na2SO3) = 24��8g��2��52g=27��32g<35��00g�������ڸù����л�����Na2SO4�����ѡ��ΪD��
��5��ͨ�����������е����ݷ�����֪30ml������ǡ������Ʒ��ȫ��Ӧʱ���������Ϊ30��16g �������0<a<0��03ʱ������������ų�����������������������㡣b=��2��688L��0��03����a=89��6a ���� a��0��03Lʱ�������������Ʒ��ȫ��Ӧ����������������b=2��688L��2S2O3��5H2O����ȡ�����������ȵļ����֪ʶ��

��ϰ��ϵ�д�
�����Ŀ

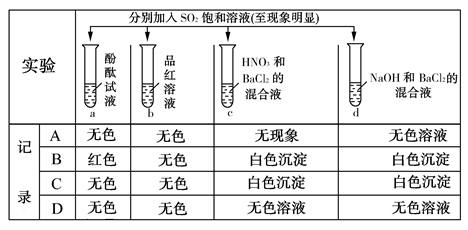
 Na2S2O3
Na2S2O3
 2NaI+Na2S4O6����Ʒ�е�Na2S2O3��5H2O�Ĵ���Ϊ��������%��
2NaI+Na2S4O6����Ʒ�е�Na2S2O3��5H2O�Ĵ���Ϊ��������%��