��Ŀ����
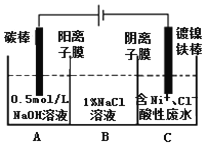
����Ŀ���Ե�ⷨ���������̲������������У����̺����ߴ�40%��50%����Ҫ�ɷ�ΪMnO2��ˮ����Һ�����������Pb2+��Sn2+���ؽ������ʣ������ղ��õ�ľм��������ά���ڽ�Ũ��������������͡�ˮ�����ɻ�ԭ�ǣ����������£�����������̷�Ӧ���ɿ����Ե������̡�

(1)��ҵ�ϵ�������̵�ˮ��Һ���������̣������ĵ缫��ӦʽΪ_____________��
(2)д��ľмˮ�ⷴӦ�Ļ�ѧ����ʽ��___________
��ƽ�������跴Ӧ����ʽC6H12O6+MnO2+H2SO4��MnSO4+CO2+H2O_________________
(3)��ȡ������������裬�����������_____________(�ѧʽ)����Ⱦ������
(4)�����յ�һ�ָ���Ʒ����Ҫ��ũҵ�������ϣ�д���仯ѧʽ____��
(5)ȡһ���������������ʵ��������õ���ͼ����������������Ϊ________mL������������������������ᵼ��____________(�ѧʽ)������������

(6)ij��������MnO2(��Է�������Ϊ87)����������Ϊ50.0%����174g����������320g36.5%��Ũ�����ϼ��ȣ�������������ڱ�״����Ӧ����_________L��(�����������е������ɷֲ����뷴Ӧ)
���𰸡�4OH--4e-=O2��+2H2O��2H2O-4e-=O2��+4H+ (C6H10O5)n+nH2O![]() n C6H12O6 C6H12O6+12MnO2+12H2SO4=12MnSO4+6CO2��+18H2O H2S��SO2 (NH4)2SO4 28 CaCO3 17.9
n C6H12O6 C6H12O6+12MnO2+12H2SO4=12MnSO4+6CO2��+18H2O H2S��SO2 (NH4)2SO4 28 CaCO3 17.9
��������
��������̿�֪��ľм�е���ά���������������ˮ�����������ǣ������Һ�м��������࣬�������ж��������������Ƿ�Ӧ���������̡�������̼��ˮ������Һ�м���̼��Ƶ�����ҺpH����������ȥ�ؽ���������ɫ�����˵õ������̺�����淋Ļ����Һ�������Һ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ������̲�Ʒ��
(1)��ҵ�ϵ�������̵�ˮ��Һ���������̣������ĵ缫��ӦΪˮʧ���ӷ���������Ӧ�����������缫��ӦʽΪ4OH--4e-=O2��+2H2O��2H2O-4e-=O2��+4H+���ʴ�Ϊ��4OH--4e-=O2��+2H2O��2H2O-4e-=O2��+4H+��
(2)ľм�ɷ�����ά�أ���Ũ���������£���ά��ˮ�����������ǣ���Ӧ�Ļ�ѧ����ʽΪ(C6H10O5)n+nH2O![]() n C6H12O6���������̾��������ԣ������Ǿ��л�ԭ�ԣ���������Һ�з�Ӧ���������̡�������̼��ˮ����ϵ����غ���ƽ��֪���ӷ���ʽΪC6H12O6+12MnO2+12H2SO4=12MnSO4+6CO2+18H2O���ʴ�Ϊ��(C6H10O5)n+nH2O
n C6H12O6���������̾��������ԣ������Ǿ��л�ԭ�ԣ���������Һ�з�Ӧ���������̡�������̼��ˮ����ϵ����غ���ƽ��֪���ӷ���ʽΪC6H12O6+12MnO2+12H2SO4=12MnSO4+6CO2+18H2O���ʴ�Ϊ��(C6H10O5)n+nH2O![]() n C6H12O6��C6H12O6+12MnO2+12H2SO4=12MnSO4+6CO2��+18H2O��
n C6H12O6��C6H12O6+12MnO2+12H2SO4=12MnSO4+6CO2��+18H2O��
(3)��ȡ������������裬��ͼ������炙��������⣬���ⱻ�������ɶ����������壬�ʴ�Ϊ��H2S��SO2��
(4)������麟�ȥ�ؽ����õ������̺�����淋Ļ����Һ�������������刺��������ʣ��ʴ�Ϊ��(NH4)2SO4��
(5)��ͼ���֪���̵Ľ���������������28mLʱ�����������������Ϊ28mL�����������������������Һ��ȸߣ��к�ʱ����̼������࣬���ӹ�ҵ���ɱ����ʴ�Ϊ��28��̼��ƣ�
(6) 174g�������к��ж������̵����ʵ���Ϊ![]() =1mol��320g36.5%��Ũ������HCl�����ʵ���Ϊ
=1mol��320g36.5%��Ũ������HCl�����ʵ���Ϊ![]() =3.2mol���ɷ�Ӧ�Ļ�ѧ����ʽ�ɵ�4HCl��Cl2��������������ڱ�״�������Ϊ
=3.2mol���ɷ�Ӧ�Ļ�ѧ����ʽ�ɵ�4HCl��Cl2��������������ڱ�״�������Ϊ![]() ��22.4L/mol��17.9L���ʴ�Ϊ��17.9��
��22.4L/mol��17.9L���ʴ�Ϊ��17.9��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʵ���������������ý��۴������ �� ��
ѡ�� | ���� | ���� | ���� |
A | �������м���Ũ���� | ���DZ�����ɶ�ĺ���״̿�����ų��д̼�����ζ������ | Ũ���������ˮ�Ժ�ǿ������ |
B | ����õ�Na2SO3��Һ�м�������Ba(NO3)2��Һ���ټ�������ϡ���� | �ȳ��ְ�ɫ������Ȼ�ֳ����ܽ� | ����Na2SO3������ |
C | ij��Һ�еμ�K3[Fe(CN)6]��Һ | ������ɫ���� | ԭ��Һ����Fe2+��������Fe3+ |
D | ���Ũ�ȵ�KCl��KI���Һ����εμ�AgNO3��Һ | �ȳ��ֻ�ɫ���� | Ksp(AgCl)>Ksp(AgI) |
A. AB. BC. CD. D
����Ŀ��I����ȩ��ľ�ļӹ���ҽҩ�ȷ�������Ҫ��;���״�ֱ�������ǹ�ҵ�Ϻϳɼ�ȩ���·������Ʊ������漰����Ҫ��Ӧ���£�
��ӦI��CH3OH(g)![]() HCHO(g)+H2(g) ��H1=+85.2kJ/mol
HCHO(g)+H2(g) ��H1=+85.2kJ/mol
��ӦII��CH3OH(g)+![]() O2(g)
O2(g)![]() HCHO(g)+H2O(g) ��H2
HCHO(g)+H2O(g) ��H2
��ӦIII��2H2(g)+O2(g)![]() 2H2O(g) ��H3=��483.6kJ/mol
2H2O(g) ��H3=��483.6kJ/mol
��1�����㷴Ӧ��ķ�Ӧ����H2=__________________��
��2�������еļ��쵰��(Mb)����O2�������MbO2��Mb(aq)+O2(g)![]() MbO2(aq)������k����k���ֱ��ʾ����Ӧ���淴Ӧ�����ʳ�����������=k����c(Mb)��P(O2)������=k����c(MbO2)��37��ʱ��ü��쵰�Ľ�϶�(��)��P(O2)�Ĺ�ϵ���±�[��϶�(��)ָ����O2��ϵļ��쵰��ռ�ܼ��쵰�İٷֱ�]��
MbO2(aq)������k����k���ֱ��ʾ����Ӧ���淴Ӧ�����ʳ�����������=k����c(Mb)��P(O2)������=k����c(MbO2)��37��ʱ��ü��쵰�Ľ�϶�(��)��P(O2)�Ĺ�ϵ���±�[��϶�(��)ָ����O2��ϵļ��쵰��ռ�ܼ��쵰�İٷֱ�]��
�ټ���37�桢P(O2)Ϊ2.00kPaʱ��������Ӧ��ƽ�ⳣ��K=_______��
P(O2) | 0.50 | 1.00 | 2.00 | 3.00 | 4.00 | 5��00 | 6.00 |
����MbO2%�� | 50.0 | 67.0 | 80.0 | 85.0 | 88.0 | 90.3 | 91.0 |
�ڵ���ƽ��ʱ���쵰����O2�Ľ�϶�(��)��O2��ѹǿ[P(O2)]֮��Ĺ�ϵʽ��=_______(�ú���k����k����ʽ�ӱ�ʾ)��
II��CO2�����������壬Ҳ����Ҫ�Ļ���ԭ�ϣ���CO2Ϊԭ�Ͽɺϳɶ����л��
��3��CO2����������ϩ����֪��2CO2(g)+6H2(g)![]() CH2=CH2(g) + 4H2O(g) ��H��QkJ/mol��һ�������£� ����ͬ��Ͷ�ϱ�X[X��
CH2=CH2(g) + 4H2O(g) ��H��QkJ/mol��һ�������£� ����ͬ��Ͷ�ϱ�X[X��![]() ]��ij�ݻ��ɱ�ĺ�ѹ�ܱ������г���CO2��H2����ò�ͬͶ�ϱ�ʱCO2��ת�������¶ȵĹ�ϵ��ͼ��ʾ��
]��ij�ݻ��ɱ�ĺ�ѹ�ܱ������г���CO2��H2����ò�ͬͶ�ϱ�ʱCO2��ת�������¶ȵĹ�ϵ��ͼ��ʾ��

��X1______X2����������������������ͬ����Q______0��
��ͼ��A��B��C�����Ӧ��ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵΪ______��
��4�������£���NaOH��Һ��CO2�����������Խ���̼�ŷţ����ҿɵõ���Ҫ�Ļ�����ƷNa2CO3��NaHCO3��
����֪25��ʱ0.1mol / L��NaHCO3��Һ��pH=8.3����ͨ������ȷ����Һ�и�����Ũ���ɴ�С��˳��Ϊ_____[��֪��������H2CO3�ĵ��볣��Ka1= 4.4��107��Ka2 = 5��1011 ]��
������5LNa2CO3��Һ��23.3gBaSO4����ȫ��ת��ΪBaCO3�������õ�Na2CO3��Һ�����ʵ���Ũ������Ϊ____��
[��֪��������Ksp(BaSO4)=1��107��Ksp(BaCO3)=2.5��106 ]��(������Һ����ı仯)