��Ŀ����
 SiO2��SO2��NaOH��H2O��±�ؼ��仯��������ѧ��ѧ�еij������ʣ����������������������Ź㷺��Ӧ�ã���ش������й����⣺
SiO2��SO2��NaOH��H2O��±�ؼ��仯��������ѧ��ѧ�еij������ʣ����������������������Ź㷺��Ӧ�ã���ش������й����⣺��1��SiԪ�������ڱ��е�λ����
��2���밴�۷е��ɸߵ��͵�˳���SiO2��SO2��NaOH��H2O���ֻ���������
��3����ҵ�����᳣���е����Ļ�ɫ�����ܵ�ԭ���ǣ��ٺ�Fe3+�ں�Br2�ۺ�Fe3+��Br2����ֻ������һ���Լ����ܷ�����������ɫ��ԭ���Լ���
A��KMnO4��Һ B��KSCN��Һ C������KI��Һ D��CCl4
��4����ҵ����Ư��Һ�Ļ�ѧ��Ӧ����ʽΪ
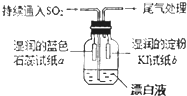
ij����С��ͬѧ������ͼ��ʾװ�����Ư��Һ�г���ͨ��SO2���壮ʵ������й۲쵽��������
�ٿ�ʼʱ����Һ�Ϸ����ְ�������ֽa��죮��ͬѧ��Ϊ��HClʹa��죬��ͬѧ��ͬ��Ĺ۵���ͬѧ����Ϊʹa������
��Ƭ�̺���Һ�Ϸ����ֻ���ɫ���壬��ֽb�����������ӷ���ʽ����b������ԭ��
��������ֽb��ɫ��ͬѧ����Ϊ���ܵ�ԭ�������֣�һ��I2�л�ԭ�ԣ�����ɫ��������ʽ���������IO3-���Ӷ�ʹ��ɫ��ʧ������
���㣺����ʵ�鷽�������,��������Ļ�ѧ����,��Ͷ�������,�Ƶ���Ҫ������
ר�⣺��������,ʵ����,Ԫ�ؼ��仯����
��������1������Ԫ���У�ԭ�Ӻ�����Ӳ���������������ȡ�����������������������ȣ�
��2��һ����˵���۷е�ߵ�˳���ǣ�ԭ�Ӿ��塢���Ӿ��塢��������ķ��Ӿ��塢��ͨ���Ӿ��壻
��3��CCl4����Һ��Ϻ�ֲ㣬�����л�����ˮ��Һ����ɫ�ж������᳣����ɫԭ��
��4������������������Һ��Ӧ��ȡƯ��Һ��
�ٶ��������ˮ��Ӧ���������ᣬ��Һ�����ԣ�Ҳ��ʹʪ�����ɫʯ����ֽ���ɫ��
�ڻ���ɫ���������������������������ɵⵥ�ʣ�����������Һ����ɫ��
�۶���������л�ԭ�ԣ��ܱ���������
��2��һ����˵���۷е�ߵ�˳���ǣ�ԭ�Ӿ��塢���Ӿ��塢��������ķ��Ӿ��塢��ͨ���Ӿ��壻
��3��CCl4����Һ��Ϻ�ֲ㣬�����л�����ˮ��Һ����ɫ�ж������᳣����ɫԭ��
��4������������������Һ��Ӧ��ȡƯ��Һ��
�ٶ��������ˮ��Ӧ���������ᣬ��Һ�����ԣ�Ҳ��ʹʪ�����ɫʯ����ֽ���ɫ��
�ڻ���ɫ���������������������������ɵⵥ�ʣ�����������Һ����ɫ��
�۶���������л�ԭ�ԣ��ܱ���������
���
�⣺��1��SiԪ�������ڱ��е�λ���ǵ������ڡ���IVA�壬�ʴ�Ϊ���������ڵ�IVA�壻
��2��һ����˵���۷е�ߵ�˳���ǣ�ԭ�Ӿ��塢���Ӿ��塢��������ķ��Ӿ��塢��ͨ���Ӿ��壬������������ԭ�Ӿ��塢NaOH�������Ӿ��塢ˮ�Ͷ����������ڷ��Ӿ��壬��ˮ�к�������������⼸�������۷е�ߵ�˳����SiO2��NaOH��H2O��SO2���ʴ�Ϊ��SiO2��NaOH��H2O��SO2��
��3��CCl4����Һ��Ϻ�ֲ㣬�л������²㣬ˮ�����ϲ㣬���л�����ɫ��ˮ��Ϊ��ɫ����Ϊ�ٺ�Fe3+���£����л���Ϊ��ɫ��ˮ��Ϊ��ɫ��Ϊ�ں�Br2���£����л���Ϊ��ɫ��ˮ��Ϊ��ɫ����Ϊ�ۺ�Fe3+��Br2���£���ѡD��
��4������������������Һ��Ӧ��ȡƯ��Һ����Ӧ����ʽΪCl2+2NaOH�TNaCl+NaClO+H2O��
���������������������ԭ��Ӧ���������HCl��
����ֽa��죬˵�����������ʴ��ڣ������������������ֽa��컹������SO2����H2SO3���йأ��ʴ�Ϊ��SO2����H2SO3����
���Ϸ����ֻ���ɫ���壬��ֽb����������Cl2+2I-�T2Cl-+I2���ʴ�Ϊ��Cl2+2I-�T2Cl-+I2��
��������ֽb��ɫ���������Ļ�ԭ�ԡ��������йأ�����ܵ�ԭ���ΪI2�������ԣ�SO2�ܽ�I2��ԭ��I-���Ӷ�ʹ��ɫ��ʧ���ʴ�Ϊ��I2�������ԣ�SO2�ܽ�I2��ԭ��I-���Ӷ�ʹ��ɫ��ʧ��
��2��һ����˵���۷е�ߵ�˳���ǣ�ԭ�Ӿ��塢���Ӿ��塢��������ķ��Ӿ��塢��ͨ���Ӿ��壬������������ԭ�Ӿ��塢NaOH�������Ӿ��塢ˮ�Ͷ����������ڷ��Ӿ��壬��ˮ�к�������������⼸�������۷е�ߵ�˳����SiO2��NaOH��H2O��SO2���ʴ�Ϊ��SiO2��NaOH��H2O��SO2��
��3��CCl4����Һ��Ϻ�ֲ㣬�л������²㣬ˮ�����ϲ㣬���л�����ɫ��ˮ��Ϊ��ɫ����Ϊ�ٺ�Fe3+���£����л���Ϊ��ɫ��ˮ��Ϊ��ɫ��Ϊ�ں�Br2���£����л���Ϊ��ɫ��ˮ��Ϊ��ɫ����Ϊ�ۺ�Fe3+��Br2���£���ѡD��
��4������������������Һ��Ӧ��ȡƯ��Һ����Ӧ����ʽΪCl2+2NaOH�TNaCl+NaClO+H2O��
���������������������ԭ��Ӧ���������HCl��
����ֽa��죬˵�����������ʴ��ڣ������������������ֽa��컹������SO2����H2SO3���йأ��ʴ�Ϊ��SO2����H2SO3����
���Ϸ����ֻ���ɫ���壬��ֽb����������Cl2+2I-�T2Cl-+I2���ʴ�Ϊ��Cl2+2I-�T2Cl-+I2��
��������ֽb��ɫ���������Ļ�ԭ�ԡ��������йأ�����ܵ�ԭ���ΪI2�������ԣ�SO2�ܽ�I2��ԭ��I-���Ӷ�ʹ��ɫ��ʧ���ʴ�Ϊ��I2�������ԣ�SO2�ܽ�I2��ԭ��I-���Ӷ�ʹ��ɫ��ʧ��
���������⿼����ʵ�鷽����ƣ���ȷ���ʵ������ǽⱾ��ؼ�����������֮��ķ�Ӧ��������ɣ���ȷ�����Ͷ���������ˮ��Һ���ܷ���������ԭ��Ӧ��ʧȥƯ���ԣ�Ϊ�״��㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�����£����и���������ָ����Һ��һ���ܴ���������ǣ�������
| A����ˮ��Һ�У�H+��I-��NO3-��SiO32- |
| B��������ˮ�У�Cl-��NO3-��Na+��SO32- |
| C��������Һ�У�H+��NH4+��Al3+��SO42- |
| D��������Һ�У�NO3-��I-��Na+��Al3+ |
��ͼ������������ʯī������1L����NaCl��Һ�У�����˵����ȷ���ǣ�������


| A����M��N�õ���ֱ������������Һ�е����̪��Һ��ʯī�缫��Χ��Һ��� |
| B��M�ӵ�Դ������N�ӵ�Դ������������������������Ϊ22.4 mL����״����ʱ��������0.001 mol NaOH |
| C��M�ӵ�Դ������N�ӵ�Դ��������ʯī�缫����Cu�缫���������Һ����CuSO4��Һ�����ʵ�������϶�ͭ |
| D��M�ӵ�Դ������N�ӵ�Դ�����������ձ�����Һ����1 L CuSO4��Һ����Ӧһ��ʱ����ձ��в�����ɫ���� |
�������ӿռ乹���������Σ������������������Σ�������Ϊ��������
| A�����������Ǽ��Է��Ӷ������ǷǼ��Է��� |
| B��NH3��������һ��δ�ɼ��Ļ��Ե��ӣ����Գɼ����ӵ��ų����ý�ǿ |
| C��NH3Ϊ������Nԭ���γ�3���ӻ������CH4��Cԭ���γ�4���ӻ���� |
| D�����ַ��ӵ�����ԭ���ӻ�������Ͳ�ͬ��NH3Ϊsp2���ӻ� |

