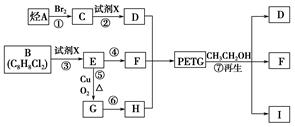
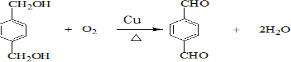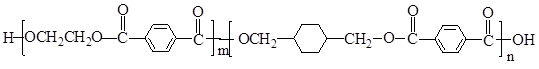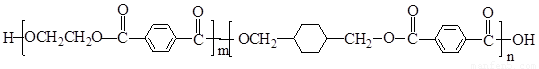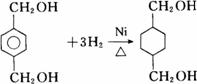��Ŀ����
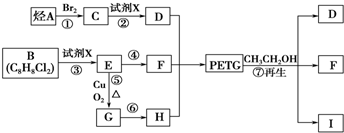
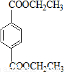
PETG��һ�����Ͳ��ϣ��ɻ������ã��Ի����������κ���в����ṹ��ʽ���£�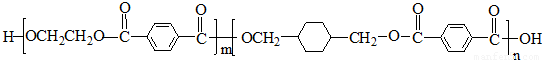
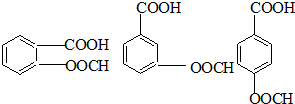
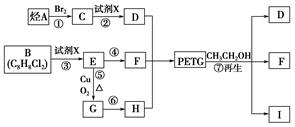
��֪��RCOOR1+R2OH-��RCOOR2+R1OH��R��R1��R2��ʾ����������������ͼ��ʾ�ĺϳ�·�߿ɺϳ�PETG��
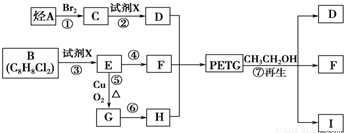
�Իش��������⣺
��1������������Ӧ�У�����ȡ����Ӧ����______����д��ţ���
��2��д���ṹ��ʽ��B______��I______��
��3��д����ѧ����ʽ����Ӧ��______����Ӧ��______��
��4���ϳ�ʱӦ���Ƶĵ�������ʵ���n��D����n��F����n��H��=______����m��n��ʾ����
��5��д��ͬʱ��������Ҫ�������H������ͬ���칹��Ľṹ��ʽ�������ڷ����廯�����ұ�����������ȡ������������NaHCO3��Һ��Ӧ����������ų������ܷ���ˮ�ⷴӦ��______��
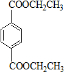
���𰸡���������PETG�Ľṹ��ʽ��֪��Ӧ�ĵ�����HOCH2CH2OH��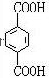 ��
��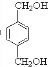 ����DӦΪHOCH2CH2OH��FΪ
����DӦΪHOCH2CH2OH��FΪ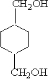 ��HΪ
��HΪ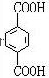 ��EΪ
��EΪ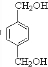 ��GΪ
��GΪ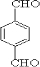 ��BΪ
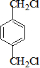
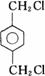
��BΪ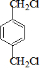 ��AΪCH2=CH2��CΪCH2BrCH2Br��IΪ
��AΪCH2=CH2��CΪCH2BrCH2Br��IΪ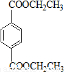 ������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
����⣺��PETG�Ľṹ��ʽ��֪��Ӧ�ĵ�����HOCH2CH2OH��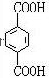 ��
��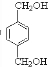 ����DӦΪHOCH2CH2OH��FΪ
����DӦΪHOCH2CH2OH��FΪ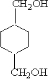 ��HΪ
��HΪ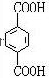 ��EΪ
��EΪ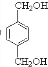 ��GΪ
��GΪ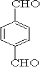 ��BΪ
��BΪ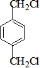 ��AΪCH2=CH2��CΪCH2BrCH2Br��IΪ
��AΪCH2=CH2��CΪCH2BrCH2Br��IΪ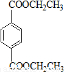 ��
��
��1�������Ϸ���������ת����ϵ��֪�ڢۢ�����ȡ����Ӧ���٢�Ϊ�ӳɷ�Ӧ������Ϊ������Ӧ��
�ʴ�Ϊ���ڢۢߣ�
��2�������Ϸ�����֪BΪ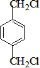 ��IΪ
��IΪ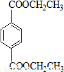 ��
��
�ʴ�Ϊ��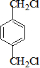 ��
�� ��
��
��3����Ӧ�ܵķ�ӦΪ ����Ӧ�ݵķ�ӦΪ
����Ӧ�ݵķ�ӦΪ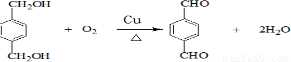 ��
��
�ʴ�Ϊ�� ��
��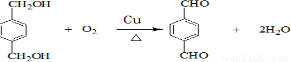 ��
��
��4���ϳ�ʱӦ���Ƶĵ�������ʵ���n ��D����n ��F����n ��H��=m��n����m+n����
�ʴ�Ϊ��m��n����m+n����
��5��HΪ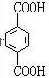 ����Ӧ��ͬ���칹�壬�����ڷ����廯�����ұ�����������ȡ������������NaHCO3��Һ��Ӧ����������ų���˵�������Ȼ������ܷ���ˮ�ⷴӦ��˵�����������������Ϊ
����Ӧ��ͬ���칹�壬�����ڷ����廯�����ұ�����������ȡ������������NaHCO3��Һ��Ӧ����������ų���˵�������Ȼ������ܷ���ˮ�ⷴӦ��˵�����������������Ϊ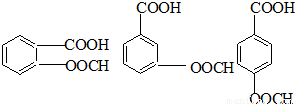 ��
��
�ʴ�Ϊ��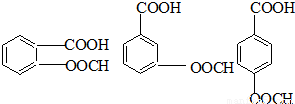 ��
��
���������⿼���л�����ƶϣ�������ѧ�������������ƶ������Ŀ��飬������Ϣ����ע����ݸ߾���Ľṹ��ʽ�жϵ���Ϊ�������ͻ�ƿڣ�����ʱע����������Ϣ��Ϊ������Ĺؼ�����Ŀ�Ѷ��еȣ�
 ��
�� ����DӦΪHOCH2CH2OH��FΪ
����DӦΪHOCH2CH2OH��FΪ ��HΪ
��HΪ ��EΪ
��EΪ ��GΪ
��GΪ ��BΪ
��BΪ ��AΪCH2=CH2��CΪCH2BrCH2Br��IΪ
��AΪCH2=CH2��CΪCH2BrCH2Br��IΪ ������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮����⣺��PETG�Ľṹ��ʽ��֪��Ӧ�ĵ�����HOCH2CH2OH��
 ��
�� ����DӦΪHOCH2CH2OH��FΪ
����DӦΪHOCH2CH2OH��FΪ ��HΪ
��HΪ ��EΪ
��EΪ ��GΪ
��GΪ ��BΪ
��BΪ ��AΪCH2=CH2��CΪCH2BrCH2Br��IΪ
��AΪCH2=CH2��CΪCH2BrCH2Br��IΪ ��
����1�������Ϸ���������ת����ϵ��֪�ڢۢ�����ȡ����Ӧ���٢�Ϊ�ӳɷ�Ӧ������Ϊ������Ӧ��
�ʴ�Ϊ���ڢۢߣ�
��2�������Ϸ�����֪BΪ
 ��IΪ
��IΪ ��
���ʴ�Ϊ��
 ��
�� ��
����3����Ӧ�ܵķ�ӦΪ
 ����Ӧ�ݵķ�ӦΪ
����Ӧ�ݵķ�ӦΪ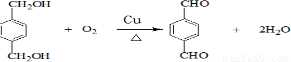 ��
���ʴ�Ϊ��
 ��
��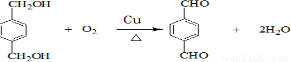 ��
����4���ϳ�ʱӦ���Ƶĵ�������ʵ���n ��D����n ��F����n ��H��=m��n����m+n����
�ʴ�Ϊ��m��n����m+n����
��5��HΪ
 ����Ӧ��ͬ���칹�壬�����ڷ����廯�����ұ�����������ȡ������������NaHCO3��Һ��Ӧ����������ų���˵�������Ȼ������ܷ���ˮ�ⷴӦ��˵�����������������Ϊ
����Ӧ��ͬ���칹�壬�����ڷ����廯�����ұ�����������ȡ������������NaHCO3��Һ��Ӧ����������ų���˵�������Ȼ������ܷ���ˮ�ⷴӦ��˵�����������������Ϊ ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶϣ�������ѧ�������������ƶ������Ŀ��飬������Ϣ����ע����ݸ߾���Ľṹ��ʽ�жϵ���Ϊ�������ͻ�ƿڣ�����ʱע����������Ϣ��Ϊ������Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ