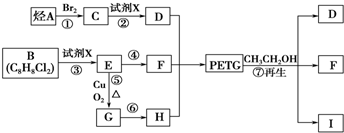
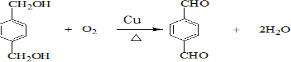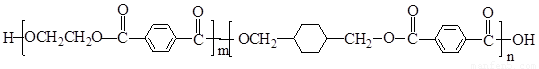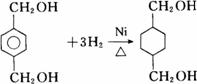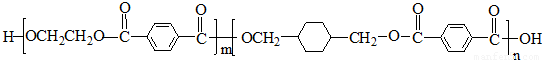��Ŀ����
PETG��һ�����Ͳ��ϣ��ɻ������ã��Ի����������κ���в����ṹ��ʽ���£�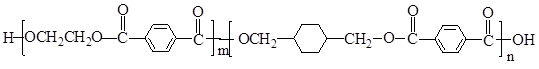
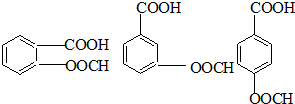
��֪��RCOOR1��R2OH�D��RCOOR2��R1OH(R��R1��R2��ʾ����)����������ͼ��ʾ�ĺϳ�·�߿ɺϳ�PETG��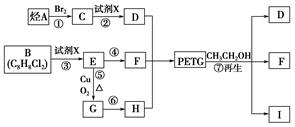
�Իش��������⣺
��1������������Ӧ�У�����ȡ����Ӧ����__________(��д���)��
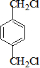
��2��д���ṹ��ʽ��B_____________________________��I_________________________��
��3��д����ѧ����ʽ��
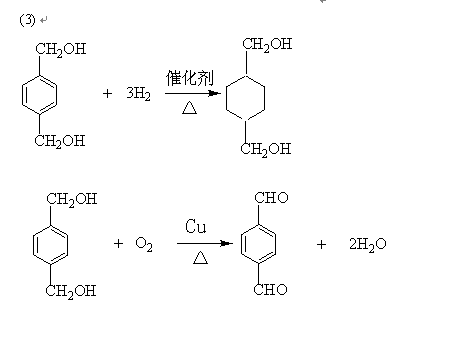
��Ӧ�� ��
��Ӧ�� ��
��4���ϳ�ʱӦ���Ƶĵ�������ʵ���n (D)��n (F)��n (H)��______________(��m��n��ʾ)��
��1���ڢۢ�
��2��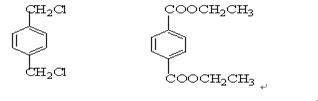
��4��m ��n ����m+n)
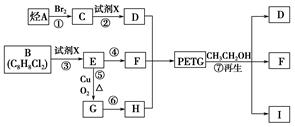
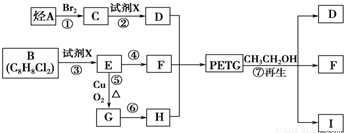
���������������������ƶϳ���AΪCH2=CH2��BΪ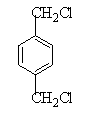 ��CΪCH2BrCH2Br��DΪHOCH2CH2OH��EΪ
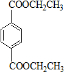
��CΪCH2BrCH2Br��DΪHOCH2CH2OH��EΪ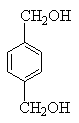 ��FΪ
��FΪ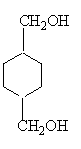 ��GΪ
��GΪ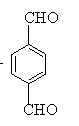 ��HΪ
��HΪ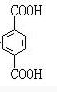
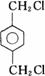
IΪ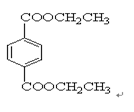 ���ʣ�1��2��3��7����ȡ����Ӧ��1��4Ϊ�ӳɷ�Ӧ������Ϊ������Ӧ����2���У�BI�Ľṹ�ֱ�Ϊ
���ʣ�1��2��3��7����ȡ����Ӧ��1��4Ϊ�ӳɷ�Ӧ������Ϊ������Ӧ����2���У�BI�Ľṹ�ֱ�Ϊ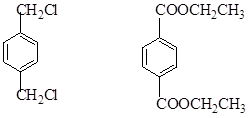 ����3���з�Ӧ�ܢݷֱ�Ϊ��
����3���з�Ӧ�ܢݷֱ�Ϊ��
��4���ϳ�ʱӦ���Ƶĵ�������ʵ���n (D)��n (F)��n (H)��m ��n ����m+n)��
���㣺�л��ƶ���
������������һ���л��ƶ��⣬�Ǹ߿�������ص㡣���и�������Ϣ���ϴ���һ�����ۺ��ԣ�����һ�����Ѷȡ�