��Ŀ����
����Ŀ�����������л������У�H2O ��Na2O ��I2 ��NaCl��KOH��CO2��NH4Cl��Na2O2������գ�
��1��ֻ�ɷǽ�����ɵ����ӻ�������________________��
��2�����м��Թ��ۼ������ӻ�������________________��
��3��ֻ���зǼ��Թ��ۼ�����______________________��
��4���������Ӽ����зǼ��Լ��Ļ�������___________________��
������A��B��C��D��E���ֶ�����Ԫ�أ������ڵ�A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ������ͼ��ʾ��E�����������������������E����������A�������Ӻ�����Ӳ�ṹ��ͬ��

��ش��������⣺
��1��д��BԪ�ص�ԭ�ӽṹʾ��ͼ_______________________��
��2��A����Ԫ�����γ�ԭ�����ʵ���֮��Ϊ1:1�Ļ���������ʽΪ______________��
��3����D��E�γɵĻ������ˮ��Һ�е���NaOH��Һֱ���������۲쵽�������ǣ�_______________�����η�Ӧ�����ӷ���ʽΪ___________________��
��4��B��C��D��E������������ˮ��������Դ�ǿ������˳��Ϊ�������û�ѧʽ��ʾ����___________________��
���𰸡� NH4Cl KOH I2 Na2O2 ![]()
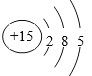 �Ȳ�����ɫ�������������ʧ Al(OH)3��OH��=AlO2����2H2O HClO4 >H2SO4 > H3PO4 > Al(OH)3
�Ȳ�����ɫ�������������ʧ Al(OH)3��OH��=AlO2����2H2O HClO4 >H2SO4 > H3PO4 > Al(OH)3
����������I��H2O�Ǻ����Լ��Ĺ��ۻ����Na2O��ֻ�����Ӽ������ӻ����I2��ֻ���Ǽ��Լ��ĵ��ʣ�NaCl��ֻ�����Ӽ������ӻ����KOH�Ǻ����Ӽ������Թ��ۼ������ӻ����CO2��ֻ�����Թ��ۼ��Ĺ��ۻ����NH4Cl�Ǻ����Ӽ������Թ��ۼ��ȵ����ӻ��������ֻ�зǽ���Ԫ����ɵ����ӻ����Na2O2�Ǻ��Ǽ��Թ��ۼ������Ӽ������ӻ����
�ʴ�Ϊ����1��NH4Cl��2��KOH NH4Cl��3��I2��4��Na2O2
��II��A��B��C��D��E���ֶ�����Ԫ�أ���ͼ��֪��AΪ�ڶ�����Ԫ�أ�B��C��DΪ��������Ԫ�أ�E�������������������������EΪ13������A��B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ���Cԭ������Ϊx����AΪx-8��BΪx-1��DΪx+1������(x-8)+(x-1)+x+(x+1)=56����x=16������AΪO��BΪP��CΪS��DΪCl��
��1�����Ϸ�����BΪP��ԭ�ӽṹʾ��ͼΪ��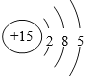 ��
��
��2��AΪO��A����Ԫ�����γ�ԭ�����ʵ���֮��Ϊ1:1�Ļ������ΪH2O2������ʽΪ��![]() ��
��
��3��D��E�γɵĻ�����Ϊ��AlCl3����AlCl3ˮ��Һ�е���NaOH��Һֱ���������������������������������ת��Ϊ�����Ե�NaAlO2�����Թ۲쵽�������ǣ��Ȳ�����ɫ�������������ʧ�����η�Ӧ���ӷ���ʽΪ��Al(OH)3��OH��=AlO2����2H2O��
��4��B��C��D��E������������ˮ����ֱ�Ϊ��H3PO4��H2SO4��HClO4��Al(OH)3��������ǿ����˳��Ϊ��HClO4 >H2SO4 > H3PO4 > Al(OH)3��


