��Ŀ����
��10�֣���1���ٸ���ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ�� ��
�ڸ�����ͼ��ʾ������ж�����˵������ȷ���ǣ� ��
A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)===CO2(g)��H2(g)������H����41kJ/mol
B���÷�ӦΪ���ȷ�Ӧ
C���÷�ӦΪ���ȷ�Ӧ
D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ/mol
��2����֪16 g��������ȫȼ��ʱ�ų�148.4 kJ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��3����ͼ��ij�¶��£�N2��H2��Ӧ�����������仯������ͼ���÷�Ӧ���Ȼ�ѧ����ʽΪ�� ��
a��b�������߲��������ԭ��ܿ����� ��
��10�֣���ÿ��2�֣�
�ٷ���ʽ�� CO��g��+H2O��g��= CO2��g��+ H2��g�� ��H = ��41 kJ/mol ��
�ڣ� BD ��
��2���Ȼ�ѧ����ʽ�� S��s��+O2��g�� = SO2��g�� ��H = ��296.8 kJ/mol��
��3���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��= 2NH3��g�� ��H = ��92 kJ/mol��
a��bԭ��ܿ����� b���˴�
����:

 ��������ѧ����ϵ�д�
��������ѧ����ϵ�д���10�֣���1���ٸ���ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ�� ��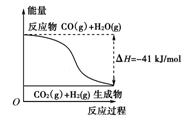
�ڸ�����ͼ��ʾ������ж�����˵������ȷ���ǣ� ��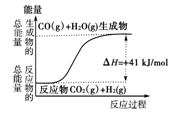
| A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)===CO2(g)��H2(g)������H����41 kJ/mol |
| B���÷�ӦΪ���ȷ�Ӧ |
| C���÷�ӦΪ���ȷ�Ӧ |
| D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ/mol |
��3����ͼ��ij�¶��£�N2��H2��Ӧ�����������仯������ͼ���÷�Ӧ���Ȼ�ѧ����ʽΪ�� ��
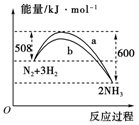
a��b�������߲��������ԭ��ܿ����� ��




