��Ŀ����
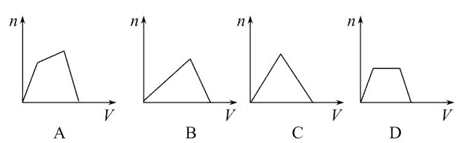
������CO2ͨ��NaOH��Ba(OH)2�Ļ��ϡ��Һ�У����ɳ��������ʵ���(n)��ͨ��CO2��������(V)�Ĺ�ϵ����ͼ��ʾ��ͼ��AB�α�ʾ�����ӷ���ʽ�Ⱥ�˳����ȷ����(����)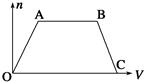
��CO2��OH��=HCO3������CO2��2OH��=CO32����H2O
��CO32����Ba2��=BaCO3������BaCO3��CO2��H2O=Ba2����2HCO3������CO32����CO2��H2O=2HCO3��
| A���ۢ� | B���ڢ� | C���ݢ� | D���ܢ� |
B
����

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�����һNa2SO3��Na2SO4�������Ʒag��Ϊ�˲ⶨ����Na2SO3��������������������·������������Բ���������
| A������Ʒ���Ƴ���ҺV1L��ȡ����25.00mL�ñ�KMnO4��Һ�ζ������ı�KMnO4��ҺV2mL |
| B������Ʒ�м�����H2O2���ټ�����BaCl2��Һ�����ˣ�������ϴ�ӡ��������������Ϊbg |
| C������Ʒ������ϡ�����ַ�Ӧ���ټ�������BaCl2��Һ�����ˣ�������ϴ�ӡ��������������Ϊcg |
| D������Ʒ������ϡ�����ַ�Ӧ�����ɵ���������ͨ��ʢ�б���NaHSO3��ϴ��ƿ��ʢ��ŨH2SO4��ϴ��ƿ��ʢ�м�ʯ�ҵĸ���ܢ�ʢ�м�ʯ�ҵĸ���ܢⶨ����ܢ�����dg |
FeS2�Ľṹ������Na2O2����һ�ֹ�������ᷴӦʱ����H2S2��H2S2�ֽ⡣ʵ������ϡ������FeS2������ϣ���Ӧ��Ϻ������ɵ�������
| A��H2S | B��S | C��FeS | D��FeSO4 |
��������CO2ͨ��������Һ�У����ܲ�����������( )��
| A��ƫ��������Һ | B��ʯ��ˮ | C������������Һ | D���Ȼ�����Һ |
�����йع輰��Ļ������������;˵��������ǣ� ��
| A��̫���ܵ�ؿɲ��ù������������Ӧ�������ڻ��������� |
| B��Na2SiO3������ľ�ķ���� |
| C������������������� |
| D����Ϊ����ʱ����������̼���Ʒ�Ӧ�ų�CO2�����Թ�������Ա�̼��ǿ |
1820��±�������MnO2��KClO3�ֽ��������������Ƶõ��������쳣����ζ����������ͨ��KI������Һ����Һ�������������п��ܻ��У� ��
| A��Cl2 | B��Br2 | C��HCl | D��CO2 |
����ÿ��ѡ���е��������ʶ��ܷ�Ӧ,���ܷų�ͬһ���������(����)
| A��ͭ��ϡ����,ͭ��Ũ���� |
| B������ϡ����,����Ũ���� |
| C��������ϡ����,����������ϡ���� |
| D��������ϡ����,������ϡ���� |
һ����X���������ƹ���,�ɷų���ɫ����Y,Y����һϵ��������������ˮ�ɵ�Z��Һ,Y��Z��Һ��Ӧ������X,��X�����������еģ� ��
| A��(NH4)2SO4 | B��NH4NO3 | C��NH4Cl | D��NH4HCO3 |