��Ŀ����
���Խ�������ԭ��Ӧ2H2��O2===2H2O��Ƴ�ԭ��ء�
(1)��������������������������Һ����ȼ�ϵ�أ���ͨ�������Ӧ��________������ͨ����������________���缫��ӦʽΪ������________________������________________��
(2)���KOH��Ϊϡ����������ʣ���缫��ӦʽΪ������ ������__________________________��
(3)(1)��(2)�ĵ����Һ��ͬ����Ӧ���к�����Һ��pH���кα仯�� ��
(4)���H2��Ϊ���飬KOH��Һ���������Һ����缫��ӦʽΪ������____________________________������________________________________��
(1)��������������������������Һ����ȼ�ϵ�أ���ͨ�������Ӧ��________������ͨ����������________���缫��ӦʽΪ������________________������________________��
(2)���KOH��Ϊϡ����������ʣ���缫��ӦʽΪ������ ������__________________________��
(3)(1)��(2)�ĵ����Һ��ͬ����Ӧ���к�����Һ��pH���кα仯�� ��
(4)���H2��Ϊ���飬KOH��Һ���������Һ����缫��ӦʽΪ������____________________________������________________________________��
(1)H2��O2��O2��2H2O��4e����4OH����H2��2OH����2H2O��2e��
(2)O2��4H����4e����2H2O��H2��2H����2e��
(3)ǰ�߱�С�����߱��
(4)2O2��4H2O��8e����8OH����CH4��10OH����CO32����7H2O��8e��
(2)O2��4H����4e����2H2O��H2��2H����2e��
(3)ǰ�߱�С�����߱��
(4)2O2��4H2O��8e����8OH����CH4��10OH����CO32����7H2O��8e��
���������(1)���ݵ�ط�Ӧʽ��֪�ڷ�Ӧ��H2��������O2����ԭ��H2Ӧ���ڸ����Ϸ�Ӧ��O2Ӧ���������Ϸ�Ӧ������Ϊ�Ǽ�����Һ����������H���μӻ����ɣ��ʸ����ĵ缫��ӦΪH2��2OH����2H2O��2e���������ĵ缫��ӦΪO2��2H2O��4e����4OH����
(2)�����������ʻ�Ϊ������Һ����ʱӦ���Dz�������OH�����ɣ��ʸ����ĵ缫��ӦΪH2��2H����2e���������ĵ缫��ӦΪO2��4H����4e����2H2O��
(3)����ǰ���ڼ��������·�Ӧ��KOH�������䣬������ʱH2O���࣬����Һ��ϡ��pH����С��������Ϊ����Һ��H2SO4�������䣬H2O���࣬����Һ��ϡ��pH�����
(4)���H2��Ϊ���飬KOH��Һ������ʣ�������Ϊ2O2��4H2O��8e����8OH������ʱ������CO2�ų�������ΪCH4��10OH����CO32����7H2O��8e����
���������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ������Ҫ�ǿ���ѧ����ԭ���ԭ������Ϥ�˽�̶ȣ������ڵ���ѧ���������������ͷ�ɢ˼ά���������ѧ��������û���֪ʶ���ʵ�����������������Ĺؼ�����ȷԭ��صĹ���ԭ����Ȼ��������������ü��ɡ�

��ϰ��ϵ�д�
 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
�����Ŀ
 Li2FeSiO4��Li2SO4��SiO2��
Li2FeSiO4��Li2SO4��SiO2�� ����
���� ������
������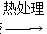 Li2FeSiO4��
Li2FeSiO4��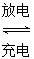 Li2FeSiO4����õ�صĸ�����________�����ʱ��������Ӧ�ĵ缫��ӦʽΪ________��
Li2FeSiO4����õ�صĸ�����________�����ʱ��������Ӧ�ĵ缫��ӦʽΪ________��




