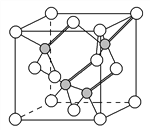
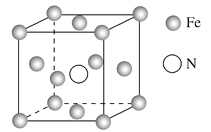
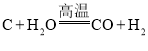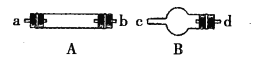ΧβΡΩΡΎ»ί
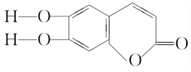
ΓΨΧβΡΩΓΩΤχΧ§ΧΰX‘Ύ±ξΉΦΉ¥Ωωœ¬ΒΡΟήΕ»ΈΣ1.16 gΓΛLΘ≠1Θ§DΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§EΈΣΨέ¬»““œ©Θ§”–ΙΊΈο÷ ΒΡΉΣΜ·ΙΊœΒ»γœ¬ΆΦΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΓΘ
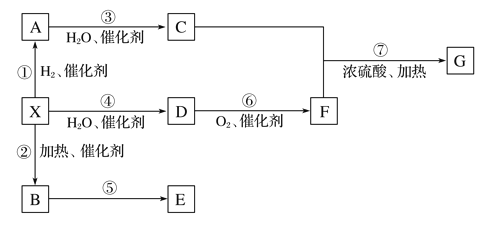
(1)Ζ¥”ΠΔΎΒΡΖ¥”Πάύ–ΆΈΣ________Θ§“ΜΕ®ΧθΦΰœ¬XΡήΖΔ…ζάύΥΤ”ΎΔίΒΡΖ¥”ΠΘ§ΥυΒΟΗΏΖ÷Ή”Μ·ΚœΈοΒΡΫαΙΙΦρ ΫΈΣ
_________________ΓΘ
(2)A”κF‘Ύ¥ΏΜ·ΦΝΉς”Οœ¬÷±Ϋ”…ζ≥…GΒΡΜ·―ßΖΫ≥Χ ΫΈΣ
_______________________________________________________________________________ΓΘ
(3)œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «________ΓΘ
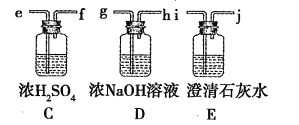
AΘ°AΓΔBΓΔEΨυΡή ΙΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΆ …Ϊ
BΘ°GΒΡΆ§Ζ÷“λΙΙΧε÷–Ρή”κΧΦΥα«βΡΤΖ¥”Π…ζ≥…CO2ΤχΧεΒΡΈο÷ ”–4÷÷
CΘ°FΓΔGΕΦΡή”κNaOH»ή“ΚΖ¥”ΠΘ§«“ΕΦ…ζ≥…ΡΤ―Έ
DΘ°Xœ»Κσ”κFΓΔH2Ζ¥”Π“≤Ω…“‘÷ΤΒΟG
ΓΨ¥πΑΗΓΩ Φ”≥…Ζ¥”Π ![]() CH2===CH2ΘΪCH3COOH
CH2===CH2ΘΪCH3COOH![]() CH3COOCH2CH3 CD
CH3COOCH2CH3 CD
ΓΨΫβΈωΓΩΧΰX‘Ύ±ξΉΦΉ¥Ωωœ¬ΒΡΟήΕ»ΈΣ1.16 gΓΛLΘ≠1Θ§ΧΰXΒΡΡΠΕϊ÷ ΝΩΈΣΘΚ1.16 gΓΛLΘ≠1ΓΝ22.4 LΓΛmolΘ≠1=26 gΓΛmolΘ≠1Θ§‘ρΧΰXΈΣCH![]() CHΘΜAΈΣCH2=CH2ΘΜCΈΣCH3CH2OHΘΜ“ρΈΣEΈΣΨέ¬»““œ©Θ§Υυ“‘BΈΣCH2=CHClΘΜ“ρΈΣDΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§Υυ“‘DΈΣCH3CHOΘΜFΈΣCH3COOHΓΘ
CHΘΜAΈΣCH2=CH2ΘΜCΈΣCH3CH2OHΘΜ“ρΈΣEΈΣΨέ¬»““œ©Θ§Υυ“‘BΈΣCH2=CHClΘΜ“ρΈΣDΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§Υυ“‘DΈΣCH3CHOΘΜFΈΣCH3COOHΓΘ
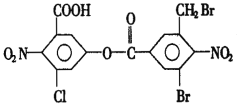
Θ®1Θ©““œ©”κHCl…ζ≥…¬»““œ© τ”ΎΦ”≥…Ζ¥”ΠΘΜCH![]() CHΖΔ…ζΦ”ΨέΖ¥”Π…ζ≥…
CHΖΔ…ζΦ”ΨέΖ¥”Π…ζ≥…![]() ΘΜΘ®2Θ©ΗυΨί‘≠Ή” ΊΚψ““œ©ΚΆ““Υα¥ΏΜ·ΦΝΧθΦΰœ¬ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…““Υα““θΞΘ§Μ·―ßΖΫ≥Χ ΫΈΣCH2=CH2ΘΪCH3COOH
ΘΜΘ®2Θ©ΗυΨί‘≠Ή” ΊΚψ““œ©ΚΆ““Υα¥ΏΜ·ΦΝΧθΦΰœ¬ΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…““Υα““θΞΘ§Μ·―ßΖΫ≥Χ ΫΈΣCH2=CH2ΘΪCH3COOH![]() CH3COOCH2CH3ΘΜΘ®3Θ©AΈΣCH2=CH2Θ§BΈΣCH2=CHClΘ§Κ§”–ΧΦΧΦΥΪΦϋΘ§Ρή ΙΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΆ …ΪΘ§EΈΣΨέ¬»““œ©Θ§≤ΜΚ§ΧΦΧΦΥΪΦϋΘ§≤ΜΡή ΙΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΆ …ΪΘ§Ι A¥μΈσΘΜCH3COOCH2CH3ΒΡΆ§Ζ÷“λΙΙΧε÷–Ρή”κΧΦΥα«βΡΤΖ¥”Π…ζ≥…CO2ΤχΧεΒΡΈο÷ ”ΠΚ§”–τ»ΜυΘ§”–CH3CH2CH2COOHΚΆ(CH3)2CHCOOHΙ≤2÷÷Θ§Ι B¥μΈσΘΜCH3COOHΚΆCH3COOCH2CH3ΕΦΡή”κNaOH»ή“ΚΖ¥”ΠΘ§«“ΕΦ…ζ≥…CH3COONaΘ§Ι C’ΐ»ΖΘΜΗυΨί‘≠Ή” ΊΚψΘ§CH
CH3COOCH2CH3ΘΜΘ®3Θ©AΈΣCH2=CH2Θ§BΈΣCH2=CHClΘ§Κ§”–ΧΦΧΦΥΪΦϋΘ§Ρή ΙΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΆ …ΪΘ§EΈΣΨέ¬»““œ©Θ§≤ΜΚ§ΧΦΧΦΥΪΦϋΘ§≤ΜΡή ΙΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΆ …ΪΘ§Ι A¥μΈσΘΜCH3COOCH2CH3ΒΡΆ§Ζ÷“λΙΙΧε÷–Ρή”κΧΦΥα«βΡΤΖ¥”Π…ζ≥…CO2ΤχΧεΒΡΈο÷ ”ΠΚ§”–τ»ΜυΘ§”–CH3CH2CH2COOHΚΆ(CH3)2CHCOOHΙ≤2÷÷Θ§Ι B¥μΈσΘΜCH3COOHΚΆCH3COOCH2CH3ΕΦΡή”κNaOH»ή“ΚΖ¥”ΠΘ§«“ΕΦ…ζ≥…CH3COONaΘ§Ι C’ΐ»ΖΘΜΗυΨί‘≠Ή” ΊΚψΘ§CH![]() CHœ»Κσ”κCH3COOHΓΔH2Ζ¥”Π“≤Ω…“‘÷ΤΒΟCH3COOCH2CH3Θ§Ι D’ΐ»ΖΘΜΉέ…œΘ§―ΓCDΓΘ
CHœ»Κσ”κCH3COOHΓΔH2Ζ¥”Π“≤Ω…“‘÷ΤΒΟCH3COOCH2CH3Θ§Ι D’ΐ»ΖΘΜΉέ…œΘ§―ΓCDΓΘ

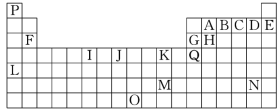
 «ßάο¬μΉΏœρΦΌΤΎΤΎΡ©Ζ¬’φ ‘ΨμΚ°ΦΌœΒΝ–¥πΑΗ
«ßάο¬μΉΏœρΦΌΤΎΤΎΡ©Ζ¬’φ ‘ΨμΚ°ΦΌœΒΝ–¥πΑΗ