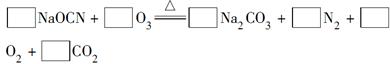��Ŀ����
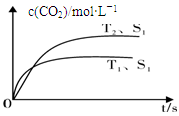
��2013���������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ������β����������Ҫԭ��Ϊ��2NO(g)+2CO(g) 2CO2(g) +N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��
2CO2(g) +N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��
�ݴ��жϣ�
��1���÷�Ӧ�Ħ�H 0��ѡ�����������������
��2������һ���¶��£���1.0 mol NO��0.5 mol CO����0.5 L�̶��ݻ��������У��ﵽƽ��ʱNO��CO��CO2��N2���ʵ����ֱ�Ϊ��0.8 mol��0.3 mol��0.2 mol��0.1 mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��ΪK= ���������¶Ȳ��䣬���������г���CO��N2��0.3 mol��ƽ�⽫ �ƶ���ѡ����������ҡ���������
���Լ���Ϊȼ�ϵ����͵�أ���ɱ�������������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ��

�ش��������⣺
��1��B��Ϊ��� �����缫��ӦʽΪ ��
��2�����ø�ȼ�ϵ������Դ����ʯī���缫���100 mL 1 mol/L������ͭ��Һ���������ռ���������������ʱ�����������ĵļ�������Ϊ ������£���
��FeS������Һ�д��ڣ�FeS(s) Fe2��(aq)��S2��(aq)��Ksp=c(Fe2��)��c(S2��),������Ksp=1.0��10��16����֪FeS������Һ��c(H��)��c(S2��)֮���������������ϵ��[c(H��)]2��c(S2��)��1.0��10��22��Ϊ��ʹ��Һ��c(Fe2��)�ﵽ1 mol/L���ֽ�����FeSͶ���䱥����Һ�У�Ӧ������Һ�е�pHΪ ��
Fe2��(aq)��S2��(aq)��Ksp=c(Fe2��)��c(S2��),������Ksp=1.0��10��16����֪FeS������Һ��c(H��)��c(S2��)֮���������������ϵ��[c(H��)]2��c(S2��)��1.0��10��22��Ϊ��ʹ��Һ��c(Fe2��)�ﵽ1 mol/L���ֽ�����FeSͶ���䱥����Һ�У�Ӧ������Һ�е�pHΪ ��
 2CO2(g) +N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��
2CO2(g) +N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��
�ݴ��жϣ�
��1���÷�Ӧ�Ħ�H 0��ѡ�����������������
��2������һ���¶��£���1.0 mol NO��0.5 mol CO����0.5 L�̶��ݻ��������У��ﵽƽ��ʱNO��CO��CO2��N2���ʵ����ֱ�Ϊ��0.8 mol��0.3 mol��0.2 mol��0.1 mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��ΪK= ���������¶Ȳ��䣬���������г���CO��N2��0.3 mol��ƽ�⽫ �ƶ���ѡ����������ҡ���������
���Լ���Ϊȼ�ϵ����͵�أ���ɱ�������������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ��

�ش��������⣺
��1��B��Ϊ��� �����缫��ӦʽΪ ��
��2�����ø�ȼ�ϵ������Դ����ʯī���缫���100 mL 1 mol/L������ͭ��Һ���������ռ���������������ʱ�����������ĵļ�������Ϊ ������£���
��FeS������Һ�д��ڣ�FeS(s)
 Fe2��(aq)��S2��(aq)��Ksp=c(Fe2��)��c(S2��),������Ksp=1.0��10��16����֪FeS������Һ��c(H��)��c(S2��)֮���������������ϵ��[c(H��)]2��c(S2��)��1.0��10��22��Ϊ��ʹ��Һ��c(Fe2��)�ﵽ1 mol/L���ֽ�����FeSͶ���䱥����Һ�У�Ӧ������Һ�е�pHΪ ��
Fe2��(aq)��S2��(aq)��Ksp=c(Fe2��)��c(S2��),������Ksp=1.0��10��16����֪FeS������Һ��c(H��)��c(S2��)֮���������������ϵ��[c(H��)]2��c(S2��)��1.0��10��22��Ϊ��ʹ��Һ��c(Fe2��)�ﵽ1 mol/L���ֽ�����FeSͶ���䱥����Һ�У�Ӧ������Һ�е�pHΪ ��I����1������2�֣�
��2��k=" 5/144" ��0.0347 ��2�֣� ����2�֣�
II����1������2�֣� CH4 + 4O2����8e��= CO2+ 2H2O����2�֣�
��2�� 1.12 L��2�֣�û��д��λ��1�֣�
III��3 ��3�֣�
��2��k=" 5/144" ��0.0347 ��2�֣� ����2�֣�
II����1������2�֣� CH4 + 4O2����8e��= CO2+ 2H2O����2�֣�
��2�� 1.12 L��2�֣�û��д��λ��1�֣�
III��3 ��3�֣�
���������I����1�����ͼ�������T1ʱ�ȵ���ƽ�⣬ƽ��ʱ������̼��Ũ��С��˵�������¶�ƽ�����ƣ���������ӦΪ���ȷ�Ӧ����H��0����2����ѧƽ�ⳣ��=������Ũ�ȵ�ϵ���η�/��Ӧ��Ũ�ȵ�ϵ���η�������k=" 5/144" ��0.0347�����������г���CO��N2��0.3 molʱ��Qc=k,ƽ�ⲻ�ƶ���II����1��ȼ�ϵ���и���ͨȼ�ϣ�����������Ӧ������ͨ������������ԭ��Ӧ�����Ը����ϼ���ʧȥ���ӽ���������γɶ�����̼��ˮ�������������õ������γ��������ӡ���2����ʯī���缫���100 mL 1 mol/L������ͭ��Һʱ�������缫��ӦΪ��4OH����4e��= O2 + 2H2O���������ǣ�Cu2++2e�� = Cu,Ȼ���ǣ�2H++2e��= H2������������amol�����ݵ����غ�ɵã�4a="0.1��2+2a," a=0.1mol��1molCH4ת�Ƶ���8mol���������ļ���0.05mol��III��������Ŀ������Ϣ��c(Fe2��)�ﵽ1 mol/Lʱ��c(S2��)= 1.0��10��16����Ϊ[c(H��)]2��c(S2��)��1.0��10��22������c(H��)= 1.0��10��3��pH=3��

��ϰ��ϵ�д�
�����Ŀ



 C��Ӧ�Ļ�ѧ����ʽ ��
C��Ӧ�Ļ�ѧ����ʽ ��