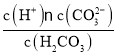��Ŀ����
����Ŀ����ҵ�Ͽ�����Ȼ��Ϊԭ���Ʊ��״���Ҳ����ˮú���ϳɼ״���
![]() ��֪��
��֪��![]() ��
��![]() ��
��![]() �������
�������![]() ��
��![]() ��ȡ�״����Ȼ�ѧ����ʽ��______��
��ȡ�״����Ȼ�ѧ����ʽ��______��
![]() ͨ�����з�Ӧ�Ʊ��״���
ͨ�����з�Ӧ�Ʊ��״���![]() ��ͼ���Ƿ�Ӧʱ
��ͼ���Ƿ�Ӧʱ![]() ��
��![]() ��Ũ����ʱ��t�ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��
��Ũ����ʱ��t�ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��![]() ��ʾƽ����Ӧ����
��ʾƽ����Ӧ����![]() ______��ƽ��ʱCO��ת��______��
______��ƽ��ʱCO��ת��______��
![]() ��һ�ݻ��ɱ���ܱ������г���
��һ�ݻ��ɱ���ܱ������г���![]() ��
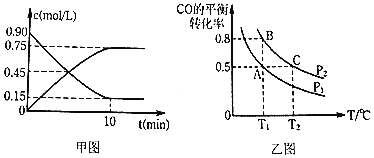
��![]() ��CO��ƽ��ת�������¶�
��CO��ƽ��ת�������¶�![]() ��ѹǿ
��ѹǿ![]() �ı仯��ͼ����ʾ��
�ı仯��ͼ����ʾ��
![]() ����˵�������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����______
����˵�������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����______![]() ����ĸ
����ĸ![]()
A ![]() ���������ʵ���
���������ʵ���![]() ���������ʵ�2��
���������ʵ�2��
B ![]() ������������ٸı�
������������ٸı�
C ��ϵ��![]() ��ת���ʺ�CO��ת�������
��ת���ʺ�CO��ת�������
D ��ϵ�������ƽ��Ħ���������ٸı�
![]() �Ƚ�A��B����ѹǿ��С
�Ƚ�A��B����ѹǿ��С![]() ______
______![]() ����
����![]() ��
��![]() ��
��![]() ��
��![]()
![]() ���ﵽ��ѧƽ��״̬Aʱ�����������Ϊ20L�������Ӧ��ʼʱ�Գ���
���ﵽ��ѧƽ��״̬Aʱ�����������Ϊ20L�������Ӧ��ʼʱ�Գ���![]() ��
��![]() 2������ƽ��״̬Bʱ�����������
2������ƽ��״̬Bʱ�����������![]() ______L��
______L��
![]() �Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ��
�Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ��![]() �缫����Ϊ���Ե缫
�缫����Ϊ���Ե缫![]()
![]() ��KOH��Һ���������ظ�����Ӧ�����ӷ���ʽΪ______��
��KOH��Һ���������ظ�����Ӧ�����ӷ���ʽΪ______��
![]() ���������Һ��KOH�����ʵ���Ϊ
���������Һ��KOH�����ʵ���Ϊ![]() ������
������![]() �״����뷴Ӧʱ���������Һ�и������ӵ����ʵ���Ũ���ɴ�С��˳����______��
�״����뷴Ӧʱ���������Һ�и������ӵ����ʵ���Ũ���ɴ�С��˳����______��
���𰸡�![]()
![]()
![]() AC
AC ![]() 4
4 ![]()
![]()
��������
��1����֪����2CH4(g)+O2(g)=2CO(g)+4H2(g) ��H=a kJ/mol����CO(g)+2H2(g)=CH3OH(g) ��H=b kJ/mol�����ݸ�˹���ɣ���+2���ڿɵã�2CH4(g)+O2(g)=2CH3OH(g) ��H=(a+2b)kJ/mol��
��2���淴Ӧ���У�COŨ�ȼ�С��CH3OHŨ��������ͼ��֪��10min����ƽ�⣬COŨ�ȱ仯Ϊ(0.9-0.15)mol/L=0.75mol/L����v(CO)=![]() =0.075mol��L-1��min-1����Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮��v(H2)=2v(CO)=0.15mol��L-1��min-1��ƽ��ʱCO��ת����=
=0.075mol��L-1��min-1����Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮��v(H2)=2v(CO)=0.15mol��L-1��min-1��ƽ��ʱCO��ת����=![]() =83.3%��
=83.3%��
��3����A��H2���������ʵ���CH3OH���������ʵ�2��������ʾ����Ӧ���ʣ���Ӧʼ�հ��ñ�����ϵ���У�����˵������ƽ�⣬��H2���������ʵ���CH3OH���������ʵ�2��ʱ����Ӧ����ƽ�⣬A����
B��H2������������ٸı��ǻ�ѧƽ����������ﵽ��ƽ�⣬B��ȷ��
C��H2��CO��ת������������ʼ���ʵ����йأ�ƽ��ʱ��һ����ȣ�����ʼ���ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ�ת����ʼ����ȣ�C����
D������������������䣬�淴Ӧ���У�������������ʵ�����С��ƽ��Ħ��������������������ƽ��Ħ���������ٸı䣬˵���ﵽ��ƽ�⣬D��ȷ��
�ʴ�Ϊ��AC��
���¶�һ��ʱ������ӦΪ���������С�ķ�Ӧ������ѹǿ��ƽ��������Ӧ�����ƶ���CO��ת��������ѹǿPA<PB��
�����ﵽ��ѧƽ��״̬Aʱ�����������Ϊ20L��COת����Ϊ50%����ת����COΪ10mol��50%=5mol����

ƽ�ⳣ��K=![]() =
=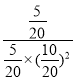 =4�������Ӧ��ʼʱ�Գ���10mol CO��20mol H2����ƽ��״̬BʱCOת����Ϊ80%����ת����COΪ10mol��80%=8mol����
=4�������Ӧ��ʼʱ�Գ���10mol CO��20mol H2����ƽ��״̬BʱCOת����Ϊ80%����ת����COΪ10mol��80%=8mol����

�����ΪV L����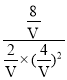 =4�����V=4��
=4�����V=4��
��4����ԭ��ظ�������������Ӧ���״��ڸ���ʧȥ���ӣ���������������̼���������ˮ�������缫��ӦʽΪ��CH3OH-6e-+8OH-=CO32-+6H2O��
�ڵ���0.75mol CH3OH���뷴Ӧʱ��������CO2��0.75mol����1mol KOH��Ӧ������Ԫ���غ㣺n(K2CO3)+n(KHCO3)=0.75mol��2n(K2CO3)+n(KHCO3)=1mol��������ã�n(K2CO3)=0.25mol��n(KHCO3)=0.5mol����Һ�ʼ��ԣ���Һ�и������ӵ����ʵ���Ũ���ɴ�С��˳���ǣ�c(K+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+)��

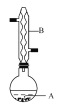
����Ŀ������������������۷���Ϣ�صijɷ�֮һ,�����㽶����ζ,ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й���������:
![]() +
+![]()
![]()
![]() +H2O
+H2O
��Է������� | �ܶ�/(g��cm-3) | �е�/�� | ˮ���ܽ��� | |
���촼 | 88 | 0.812 3 | 131 | �� |
���� | 60 | 1.049 2 | 118 | �� |
���������� | 130 | 0.867 0 | 142 | ���� |
ʵ�鲽��:
��A�м���4.4 g���촼��6.0 g���ᡢ����Ũ�����2��3Ƭ���Ƭ,��ʼ��������A,����50 min,��ӦҺ�������º����Һ©����,�ֱ�������ˮ������̼��������Һ ��ˮ ϴ��,�ֳ��IJ������������ˮMgSO4����,����Ƭ��,���˳�ȥMgSO4����,��������,�ռ�140��143�����,������������3.9 g���ش���������:
(1)����B��������________������������____________________��
(2)��ϴ�Ӳ�����,��һ��ˮϴ����ҪĿ��___________________________
�ڶ���ˮϴ����ҪĿ����_______________________________________��
(3)��ϴ�ӡ���Һ������,Ӧ�����,Ȼ����,���ֲ��____(����)��
a.ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
b.ֱ�ӽ������������ӷ�Һ©�����¿ڷų�
c.�Ƚ�ˮ��ӷ�Һ©�����¿ڷų�,�ٽ��������������¿ڷų�
d.�Ƚ�ˮ��ӷ�Һ©�����¿ڷų�,�ٽ��������������Ͽڵ���
(4)��ʵ���м�����������Ŀ����_____________________________��
(5)ʵ���м���������ˮMgSO4��Ŀ����___________________________��
(6)�����������,����ѡ��װ����ȷ����____(����)��
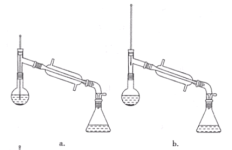
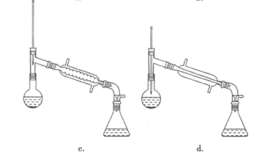
(7)��ʵ��IJ�����____(����)��
a.30% b.40% c. 60% d.90%
(8)�ڽ����������ʱ,����130��㿪ʼ�ռ����,��ʹʵ��IJ���ƫ____(��ߡ��͡�),��ԭ����____________________________________��