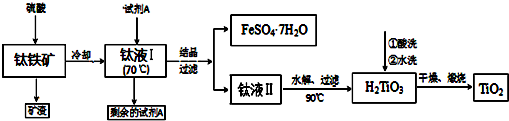
��Ŀ����
17����1����ͼ1��һ�ַ���ʽΪC4H8O2���л���ĺ������ͼ������л�����ܵĽṹ��ʽΪCH3COOCH2CH3��CH3CH2COOCH3����2����֪1������2�����Ľṹ��ʽ���£�
1������CH3CH2CH2OH 2������

��ͼ2������������������һ�ֵĺ˴Ź����ף����ҷ�����ȷֱ�Ϊ1��1��6��
��ָ���ú˴Ź����ױ�ʾ��������2-������
��3��ij�л�������A����Է�����������110��С��150����������֪������̼�������������֮��Ϊ52.24%������Ϊ������ش�
�ٸû��������Է���������134��
�ڸû�����Ļ�ѧʽ��C5H10O4��
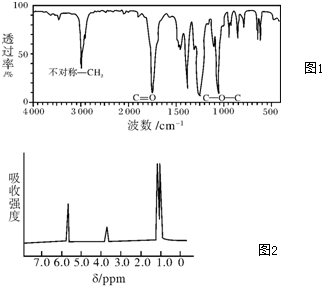
���� ��1���ɺ������ͼ�ɿ����÷������в��Գ�CH3����˸÷�������2��CH3����ͼҲ���Կ�������C=O˫����C-O-C��������дA�Ľṹʽ���ݴ˽��
��2�����ݸ��л���ĺ˴Ź������еķ�����ȿ���ȷ�����л�������к��еIJ�ͬ������ԭ�ӵ�����������ȷ���л���Ľṹ��ʽ��
��3�����ݺ���������Է��������ķ�Χ���ó���ԭ�����ķ�Χ������ȷ�������к��е���ԭ����Ŀ��������Ԫ�ص����������������к��е���ԭ����Ŀ����л����������Է���������������Է��������ͷ����к��е���ԭ����Ŀ���㻯ѧʽ��
��� �⣺��1���ɺ������ͼ�ɿ����÷������в��Գ�CH3����˸÷�������2��CH3����ͼҲ���Կ�������C=O˫����C-O-C����������A�Ľṹ��ʽΪCH3COOCH2CH3����CH3CH2COOCH3���ʴ�Ϊ��CH3COOCH2CH3��CH3CH2COOCH3��
��2�����ݸ��л���ĺ˴Ź������еķ�����ȷֱ�Ϊ1��1��6��֪�����л�������к��еIJ�ͬλ����ԭ����3�֣��ֱ�����ԭ����֮��Ϊ1��1��6��1-������CH3-CH2-CH2-OH}�в�ͬλ�õ���ԭ�������֣�����Ŀ֮��Ϊ1��3��4��
2-���� �в�ͬλ�õ���ԭ�������֣�����ԭ����Ŀ֮��Ϊ1��1��6��������������������Ϊ��2-������
�в�ͬλ�õ���ԭ�������֣�����ԭ����Ŀ֮��Ϊ1��1��6��������������������Ϊ��2-������
�ʴ�Ϊ��2-������
��3����������֪��������������Ϊ47.76%�����л����������Է�����������110��С��150��
����������ԭ�Ӹ���Ϊ����$\frac{110��0.4776}{16}$=3.28��С��$\frac{150��0.4776}{16}$=4.48��������ԭ��Ϊ4����
��������ԭ��Ϊ4����������������Ϊ47.76%�����л��������������=$\frac{4��16}{0.4776}$=134��
�ʴ�Ϊ��134��
�ڷ�������ԭ��Ϊ4��������C��H�����ԭ������֮��Ϊ��134-16��4=70����ȷ����ѧʽΪC5H10O4���ʴ�Ϊ��C5H10O4��
���� ���⿼�����л������ʽ��ȷ������Ŀ�Ѷ��еȣ�����ȷ����ԭ����Ϊ������Ĺؼ���

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�| A�� | AgNO3 | B�� | FeBr2 | C�� | NaOH | D�� | Na2CO3 |
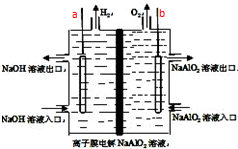 ��ҵ����������Ĥ���ƫ��������Һ�Ʊ���Ʒ�ʵ�����������װ����ͼ��ʾ��a��bΪ��Դ�ĵ缫�����й�˵����ȷ���ǣ�������
��ҵ����������Ĥ���ƫ��������Һ�Ʊ���Ʒ�ʵ�����������װ����ͼ��ʾ��a��bΪ��Դ�ĵ缫�����й�˵����ȷ���ǣ�������| A�� | ����0.1mol ���ӷ���ת��ʱ��a�����������ڱ�״����Ϊ112mL | |
| B�� | �õ��ʹ��ʱӦѡ�������ӽ���Ĥ | |
| C�� | ��ع�������������������۾�����Al��OH��3�������� | |
| D�� | �õ�ص��ܷ�Ӧ����ʽΪ��4NaAlO2+10H2O$\frac{\underline{\;ͨ��\;}}{\;}$ 4Al��OH��3��+4NaOH+O2��+2H2�� |
| ѡ�� | ���ᴿ������ | �����Լ� | ���뷽�� |
| A | ���飨��ϩ�� | ��ˮ | ��Һ |
| B | ������Һ��NaCl�� | ˮ | ���� |
| C | CH3CH2OH��CH3COOH�� | CaO | ���� |
| D | CO2��SO2�� | Na2CO3��Һ | ϴ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | O2��I2��Hg | B�� | ����Ͻ��������ʯ | ||
| C�� | Na��K��Rb | D�� | SiC�������ơ�SO2 |
| A�� |  | B�� |  | C�� |  | D�� | 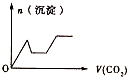 |
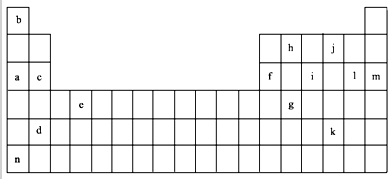
��1������Ԫ�صĵ��ʿ����ǵ�����������acdefhn������ĸ���ţ���
��2��������һЩ��̬��̬ԭ�ӵĵ�һ�����������ļ������ܣ�kJ•mol-1����
| � | X | Y | |
| ��һ������ | 519 | 502 | 580 |
| �ڶ������� | 7296 | 4570 | 1820 |
| ���������� | 11799 | 6920 | 2750 |
| ���ĵ����� | 9550 | 11600 |
�ڱ���Y����Ϊ����13��Ԫ���е�Al����Ԫ�ط��ţ�Ԫ�أ���Ԫ�ط��ű�ʾX��j��ԭ�Ӹ���1��1�γɻ�����ĵ���ʽ
 ��
����3�����ݹ���ԭ�����õ����Ų�ʽ��ʾe�ĺ�������Ų�ʽ1s22s22p63s23p63d24s2��
��4������13��Ԫ���У�Ar����Ԫ�ط��ţ�Ԫ��ԭ��ʧȥ�����һ��������Ҫ��������࣮
��5��a��c��f����Ԫ�ص�����������ˮ�����Լ��ԣ������ǿ��ΪNaOH��Mg��OH��2��Al��OH��3���û�ѧ�������𣩣�f��������������ԣ�д������NaOH��Һ��Ӧ�����ӷ���ʽ2OH-+Al2O3=AlO2-+2H2O��
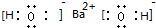 ��
��