��Ŀ����
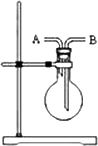
��.������ͼ��ʾװ������ȡNO2���壬ʵ�鲽�����£�![]()
��1�������Լ�飺��ش��װ�������Լ�����ķ�����
_____________________________________________________________________��
��2����������Լ�CuƬ������K���γ�ע��������������һСƬCuƬ��ע��������װ�ϲ�ѹ���ײ����ų�����Ŀ�����
��3����ȡŨHNO3����ע�������쵼�ܲ���ŨHNO3�У���������ע����������ȡ������ŨHNO3��Ȼ��Ѹ�ٹرշ���K����ش��ʱ���ܹ۲쵽��ʵ������______________________________________________________________�������ɵ�NO2��������ӽ����̶�ʱ��Ѹ�ٴ���K������ӦҺ����NaOH��Һ�к�ر�K��
��.NO2����ʵ��
��1���۲��ռ����������____________ɫ������������ѹ�����������������ɫ�ȱ�����dz��ԭ����__________________________________________________________________��
��2��Ϊ����֤����ʵ�飨2����ע�����е����壬�����һʵ�鷽��������֤��
______________________________________________________________��
��.��ʢ��a mol NO2��b mol NO���������Թܵ�����ˮ���У���ͨ��c mol O2����ַ�Ӧ���Թ���ʣ������ijɷּ����ʵ���������a��b��c�Ĺ�ϵ��������������
a��b��c�Ĺ�ϵ | ʣ������ijɷ� | ʣ����������ʵ��� |
4c=a+3b |
|
|
|
| (a+3b-4c)/3 |
| O2 |
|
��.��1���ȴ���K�����벿�ֿ�����ϵ����K�����¿̶ȣ�Ȼ���ƻ���ע�������������ֺ�ע���������Ƿ�ԭ�����ȴ���K�����벿�ֿ������رջ���K��Ȼ��������סע�����п����IJ��֣��������Ƿ����ƣ�����Ƭ�̺��ٿ������Ƿ�ԭ��
��3��ŨHNO3��CuƬ���ҷ�Ӧ�����ܽ⣬������������ɫ���壬��Һ����ɫ����ɫ����ɫ����ע����������������
��.��1������ ��ʼʱ�����С��NO2Ũ��������ɫ��������ڿ��淴Ӧ2NO2![]() N2O4����ѹ��ƽ��������Ӧ�����ƶ�������ɫ�ֱ�dz�����Ա�ԭ����
N2O4����ѹ��ƽ��������Ӧ�����ƶ�������ɫ�ֱ�dz�����Ա�ԭ����
��2������K���ų�ע������ˮ��Һ��Ȼ����ע�����������벿�ֿ�������ע�����ڵ�����Ѹ�ٱ�Ϊ����ɫ����֤��NO2��ˮ��Ӧ���ɵ�����ΪNO
��.���±�
a��b��c�Ĺ�ϵ | ʣ������ijɷ� | ʣ����������ʵ��� |
| �� | 0 |
4c��a+3b | NO |
|
4c��a+3b |
| ��4c-a-3b��/4 |
������������Ҫ������NO2����ȡ��NO2�����ʼ�NOx���йؼ��㡣��.ŨHNO3��Cu��Ӧ��ʵ�������ǣ�CuƬ���ܽ⣬������������ɫ���壬��Һ����ɫ����ɫ����ɫ����ע���������������ơ���.ѹ��NO2���壬NO2Ũ��������ɫͻȻ��������ڿ��淴Ӧ2NO2![]() N2O4����ѹ��ƽ��������Ӧ�����ƶ�������ɫ�ֱ�dz�����Ա�ԭ�����.����4NO2+O2+2H2O====4HNO3��4NO+3O2+2H2O====4HNO3 a mol NO2��b mol NO��ͨ��c mol O2��ϣ���4c=a+3b����ʣ�����壻��4c��a+3b��ʣ��NO��n��NO��=
N2O4����ѹ��ƽ��������Ӧ�����ƶ�������ɫ�ֱ�dz�����Ա�ԭ�����.����4NO2+O2+2H2O====4HNO3��4NO+3O2+2H2O====4HNO3 a mol NO2��b mol NO��ͨ��c mol O2��ϣ���4c=a+3b����ʣ�����壻��4c��a+3b��ʣ��NO��n��NO��=![]() ����4c��a+3b��ʣ��O2��n��O2��=
����4c��a+3b��ʣ��O2��n��O2��=![]() ��
��

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�