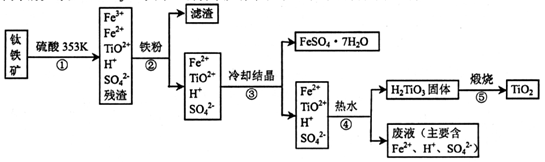
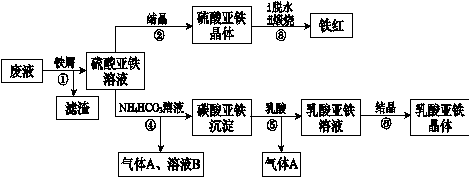
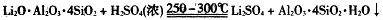��Ŀ����
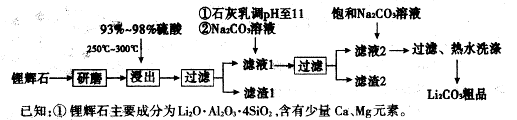
����������SnSO4�������ڶ�����ҵ��ijС�����SnSO4�Ʊ�·��Ϊ��
�������ϣ�
�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ�������
��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����
��1����ԭ�ӵĺ˵����Ϊ50����̼Ԫ��ͬ����A�壬��λ�����ڱ��ĵ� ���ڣ�1�֣�
��2���������� ���ˡ�ϴ�ӵȣ�2�֣�
��3���ܽ�SnCl2��ĩ���Ũ���ᣬԭ����
��4������Sn�۵��������������ٵ�����ҺpH ��
��5����Ӧ��õ�������SnO���õ��ó��������ӷ�Ӧ����ʽ��
��6�����������£�SnSO4��˫��ˮȥ��Ӧ�����ӷ���ʽ��
��7����С��ͨ�����з����ⶨ�������۵Ĵ��ȣ����ʲ����뷴Ӧ����
�ٽ��������������У������ķ�ӦΪ��Sn+2HCl�TSnCl2+H2����
�ڼ��������FeCl3��
������֪Ũ�ȵ�K2Cr2O7�ζ������ɵ�Fe2+���ټ������۵Ĵ��ȣ�����ƽ����ʽ��
FeCl2 + K2Cr2O7 + HCl = FeCl3 + KCl + CrCl2+ 
32.��16�֣���1���������ڣ�1�֣��� ��2������Ũ������ȴ�ᾧ��2�֣���
��3�� ����Sn2+ ��ˮ�⣨2�֣��� ��4����ֹSn2+ ��������2�֣���
��5��SnCl2 + Na2CO3=" SnO��+" CO2��+ 2NaCl��3�֣�δд���͡����Ź���1�֣�δ��ƽ��1�֣�
��6��Sn2+ + H2O2 +2H+ = Sn4 + + 2H2O ��3�֣�δ��ƽ��1�֣�
��7���� 6 1 14 6 2 2 7 H2O��3�֣���ѧʽH2O��1�֣�ϵ��ȫ��2�֣�
�����������: �⣺��1����Ԫ����̼Ԫ������ͬһ���壬���ڢ�A�壬ԭ�Ӻ˵����Ϊ50����50-2-8-8-18=14����Sn���ڵ������ڡ�
��2��������ͼ��֪���������Ǵ���Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ���
��3������Ϣ��֪��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����������ˮ��ƽ��SnCl2+H2O Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⡣
Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⡣
��4������Ϣ��֪��Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
��5����Ӧ��õ�������SnO��SnԪ�ػ��ϼ�Ϊ�仯�����ڷ�������ԭ��Ӧ��ͬʱ�������壬������Ϊ������̼����Ӧ����ʽΪ��SnCl2 + Na2CO3=" SnO��+" CO2��+ 2NaCl��
��6�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ���Sn2+�ױ�����ΪSn4+����������ԭΪˮ�����ӷ���ʽΪ��Sn2++H2O2+2H+�TSn4++2H2O��
��7����Ӧ������HԪ�أ������������������������ΪH2O�����Ը��ݵ��ǵ����غ���ƽ�ķ���ʽΪ��6FeCl2+K2Cr2O7+14HCl�T6FeCl3+2KCl+2CrCl3+7H2O
���㣺���⿼����ǻ��������⡣

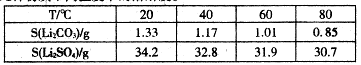
(1)ij����ÿ��Ҫ�յ�����1.6%����ú200 t,�ŷų���SO2��������Ⱦ����,������Ϊ��,����ЩSO2��������,��ô������ÿ��(��365 d��)������98%��Ũ������������;
(2)��Ҫ�����Ƽ��������,����Ӧ���Դ����������������,���Һ��������������Һ,��ƹ�������������Һ��Ũ�Ȼ���������(�������С�����䡱);
(3)��ҵ������ˮ�ࡢ����ʱ��Ҫ�õ���ԭ������������(����),����ѡԭ���Ʋ����Ļ�ѧ����ʽ���� ��;
| A������ | B����ʯ�� | C��ʯ��ʯ | D����� |
(5)��������ˮ��ԭ����
(�����ӷ���ʽ��ʾ);������ʱӲ�ȵ�ˮ�г�ȥMg2+�ķ������� ��
�� (�û�ѧ����ʽ��ʾ)��
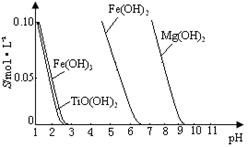
��ҵ�����÷���м(����������������������)������ʽ������[Fe(OH)SO4]�Ĺ����������£�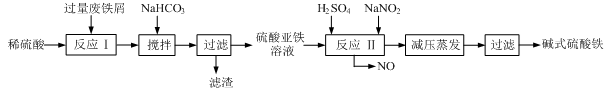
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�ش��������⣺
��1�������������м��Ŀ���� ����ʱ��Һ�д��ڵ���������Ҫ�� ����NaHCO3������ҺpHʱ�����ӷ���ʽ�� ��
��2����ʵ�������У���Ӧ���г�ͬʱͨ��O2�Լ���NaNO2��������ͬʱͨ��O2�������� ��
��3����ʽ����������ˮ���ܵ������[Fe(OH)]2+���ӣ�д��[Fe(OH)]2+����ˮ�ⷴӦ����Fe(OH)3�����ӷ���ʽ ��
��4����֪����м����Ԫ�ص���������Ϊ84.0%����������ÿ����Ӧ����Ԫ�ص���ģ�����100�ַ���м��������������� �ּ�ʽ��������
���ڿ��淴ӦN2(g)��3H2(g) 2NH3(g)����H<0������˵����ȷ����(����)��
2NH3(g)����H<0������˵����ȷ����(����)��
| A���ﵽƽ��ʱ��Ӧ���������Ũ��һ����� |
| B���ﵽƽ�����백�������´ﵽƽ��ʱ��������Ũ�ȱ�ԭƽ��ʱ�� |
| C���ﵽƽ��ʱ�������¶ȼӿ������ȷ�Ӧ�����ʣ������˷��ȷ�Ӧ�����ʣ�����ƽ�����淴Ӧ�ķ����ƶ� |
| D����������������̵���ƽ���ʱ�䣬������Ϊ�ӿ�������Ӧ�����ʣ����������淴Ӧ������ |