��Ŀ����
��4�֣����������У�4�۵������������������л�ԭ�ԣ��������Լ���������ˮ��������Һ��NaOH��Һ���Ȼ�����Һ��ϡ���ᡣҪ֤���������ƾ��л�ԭ�ԣ������������Ӧѡ�õ��Լ��� �������������� ����Ӧ�����ӷ���ʽ ��
��3�֣�A��B��C��������ѧ��������ɫ���ʣ�Ħ�����������������Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ������д��A��B��C��ˮ��Ӧ�Ļ�ѧ����ʽ��
��1��A+H2O
��2��B+H2O
��3��C+H2O
��3�֣�A��B��C��������ѧ��������ɫ���ʣ�Ħ�����������������Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ������д��A��B��C��ˮ��Ӧ�Ļ�ѧ����ʽ��
��1��A+H2O
��2��B+H2O
��3��C+H2O

��

��ϰ��ϵ�д�
�����Ŀ
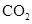
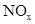 �ڴ���������������N2
�ڴ���������������N2
 �����ᷴӦ�������ռӦ����
�����ᷴӦ�������ռӦ���� ���������������ӵĸ�����Ϊ2��1�����Ƴ�
���������������ӵĸ�����Ϊ2��1�����Ƴ� ���������������ӵĸ�����Ϊ1��1
���������������ӵĸ�����Ϊ1��1 ����Ӧ���Ƴ���
����Ӧ���Ƴ��� ����ӦҲ�ܷ���
����ӦҲ�ܷ���