��Ŀ����
δ���Ľ�ͨ���߽�������һ�����ǽ��������Ƴɵ���ˮ�䷢����������������о��ý϶���ǻ�����G����1��G�����ɻ�����E��������Ԫ�أ���NH3��Ӧ�����ɣ�ͬʱ����HCl���壬G����Է�������Ϊ140�������й�Ԫ�ص���������Ϊ60%������Ԫ��J�����ƶϣ�
�ٻ�����G�Ļ�ѧʽΪ________________________________________��
����1 mol NH3��0.75 mol Eǡ����ȫ��Ӧ������E�Ļ�ѧʽΪ___________________��
��2��Ϊ��ʹ������G�ܳ�Ϊ��һ���������������ϣ��ֲ��ó�ѹ���������������������������ս��Ƴ�һ�ָ�ǿ�ȡ���Ӳ�ȡ���ĥ�𡢿���ʴ���մɲ��ϣ�������Ʒ���С����ס���Sialon������ѧͨʽ�ɱ���ΪSi6-xAlxOxJ8-x���ڽӽ��� 1
��1����Si3N4 ��SiCl4
��2��w��Al��=27x/��280+x��%
��x=2ʱ��w��Al��=19.14%����x=4ʱ��w��Al��=38.00%��
�������¶����ߣ�x����w��Al����֮����

��ϰ��ϵ�д�
�����Ŀ
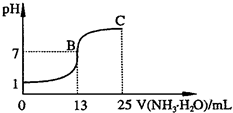 ��2011?�ൺģ�⣩���Ļ�������ijЩ�����а�������Ҫ�Ľ�ɫ��
��2011?�ൺģ�⣩���Ļ�������ijЩ�����а�������Ҫ�Ľ�ɫ��