��Ŀ����
������ʾ�����������ڴ����а���ȼ�գ���ȼ�ղ���Ի���û����Ⱦ����
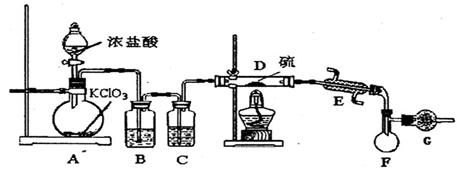
ijѧУ��ѧС���ѧ�������ͼװ��(ͼ�����еȼг�װ������ȥ)�����а����������ڲ�ͬ�����·�Ӧ��ʵ�顣

(1) ��װ��A��ȡ����������İ��������Թ���̼���εĻ�ѧʽ�� ��
��ʯ�ҵ������� ��
(2) �������İ��������������ͨ��װ��B (����Ϊ��ʯ��)�У��þƾ���Ƽ��ȣ�
���������Ļ�ѧ����ʽ�� ��
�Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(3) ��������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У�����b���϶�
��ȼ�����������ڴ�����ȼ�յĻ�ѧ����ʽ�� ��
ijѧУ��ѧС���ѧ�������ͼװ��(ͼ�����еȼг�װ������ȥ)�����а����������ڲ�ͬ�����·�Ӧ��ʵ�顣

(1) ��װ��A��ȡ����������İ��������Թ���̼���εĻ�ѧʽ�� ��
��ʯ�ҵ������� ��
(2) �������İ��������������ͨ��װ��B (����Ϊ��ʯ��)�У��þƾ���Ƽ��ȣ�
���������Ļ�ѧ����ʽ�� ��
�Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(3) ��������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У�����b���϶�
��ȼ�����������ڴ�����ȼ�յĻ�ѧ����ʽ�� ��
(1) (NH4)2CO3 ����NH4HCO3��1�֣��� ����ˮ�Ͷ�����̼
(2) 4NH3��5O2
 4NO��6H2O 2NO��O2===2NO2
4NO��6H2O 2NO��O2===2NO2(3) 4NH3��3O2
 2N2��6H2O
2N2��6H2O�����������1��Aװ�����Ʊ�藺�������Ҫ��Σ�����Ӧ��Ϊ̼��粒��塣��ʯ�ҵ�����������̼��立ֽ���ˮ�Ͷ�����̼����2����������������һ��������ˮ�������Ϊ����ɫ˵�������˶���������3������ڴ�������ȼ�գ������ɵIJ����ǵ�����ˮ��
�����������漰���˰����Ļ�ѧ���ʣ����Ʊ������Ĺ����������˹�����μ��ȷֽ�����ʣ�����װ����Ҫѧ���������ա������Ĵ���������Ҫע�ⷽ��ʽ����д���Լ���������ʡ�

��ϰ��ϵ�д�
�����Ŀ



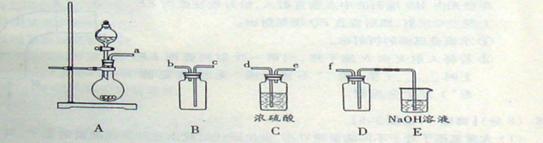
 R-Br+H2O ��
R-Br+H2O �� ���������ϡ��� �����������110�桫140������Ӧ�����ɵõ�S2C12���� S���۵�Ϊ112��8�桢�е�Ϊ444��6�棻S2C12���۵�Ϊ
���������ϡ��� �����������110�桫140������Ӧ�����ɵõ�S2C12���� S���۵�Ϊ112��8�桢�е�Ϊ444��6�棻S2C12���۵�Ϊ 76�桢�е�Ϊ138�档
76�桢�е�Ϊ138�档 2SCl2���� S2C12��ˮ������Ӧ������H2S��SO2��H2SO3��H2SO4�ȡ���ClO3-+5Cl-+6H
2SCl2���� S2C12��ˮ������Ӧ������H2S��SO2��H2SO3��H2SO4�ȡ���ClO3-+5Cl-+6H =3C12��+3H2O ��ش��������⣺
=3C12��+3H2O ��ش��������⣺