��Ŀ����
(14��)����Ũ���ᷨ(�ⶨ��Ӧ�������������)�ⶨijͭ���Ͻ���ͭ���������������ϱ������ٷ�Ӧ�г�����NO2�������������NO���ɣ��ڳ�����N02��N2O4��ϴ��ڣ��ڵ���0��ʱ����ֻ����ɫN2O4Һ�������ڡ�Ϊ��ɲⶨ����֤��NO���ɣ����������ͼʵ��װ�á�
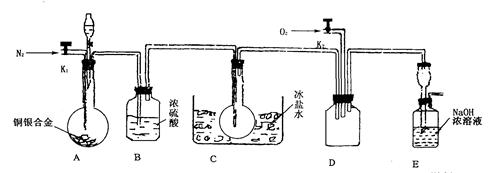
(1)ʵ�鿪ʼǰҪ�ȴ�A���ֵĻ���K1������ͨһ��ʱ��ĵ����ٹر�K1����������Ŀ����_____________________________________________________________��
(2)װ��Bƿ��������__________________________________________________��
(3)A�з�Ӧֹͣ��D�еĻ���K2��ͨ������������Ӧ��ȷ��NO��������D��Ӧ���ֵ�������________________��ʵ�鷢�֣�ͨ�������¶ȵĸߵͶ�ʵ�������нϴ�Ӱ�죬��Ϊ�˱��ڹ۲�Ӧͨ��_______________(��䡱���ȡ�)��������
(4)Ϊ�˼��ٲ�������A�з�Ӧ��ɺ�D�г��������Ӧ�������еIJ�����__________________________________________________________��
(5)ʵ�����������ݣ�����ͭ���Ͻ�����Ϊ15��0 g��Ũ��������Ϊ40 mL��Ũ��Ϊ13��5mol��L��1��ʵ���A����Һ���Ϊ40mL��H+Ũ��Ϊ1.0mol��L��1�����跴Ӧ��������ӷ�Ҳ�ֽ⣬��
�ٲμӷ�Ӧ����������ʵ���Ϊ______________��
�����Ѳ����Ӧ��Eװ�õ������ﺬ��Ԫ�ص���������Ϊȷ���Ͻ���ͭ����������������Ҫ�ⶨ��������__________��
(6)ʵ����ֻ�ⶨͭ����������������֤NO�IJ���������ͭ���Ͻ������ᷴӦ��ֻ���ʵ������Ϳ��ԴﵽĿ�ģ������ʵ�����________________________��

(1)ʵ�鿪ʼǰҪ�ȴ�A���ֵĻ���K1������ͨһ��ʱ��ĵ����ٹر�K1����������Ŀ����_____________________________________________________________��
(2)װ��Bƿ��������__________________________________________________��
(3)A�з�Ӧֹͣ��D�еĻ���K2��ͨ������������Ӧ��ȷ��NO��������D��Ӧ���ֵ�������________________��ʵ�鷢�֣�ͨ�������¶ȵĸߵͶ�ʵ�������нϴ�Ӱ�죬��Ϊ�˱��ڹ۲�Ӧͨ��_______________(��䡱���ȡ�)��������
(4)Ϊ�˼��ٲ�������A�з�Ӧ��ɺ�D�г��������Ӧ�������еIJ�����__________________________________________________________��
(5)ʵ�����������ݣ�����ͭ���Ͻ�����Ϊ15��0 g��Ũ��������Ϊ40 mL��Ũ��Ϊ13��5mol��L��1��ʵ���A����Һ���Ϊ40mL��H+Ũ��Ϊ1.0mol��L��1�����跴Ӧ��������ӷ�Ҳ�ֽ⣬��
�ٲμӷ�Ӧ����������ʵ���Ϊ______________��
�����Ѳ����Ӧ��Eװ�õ������ﺬ��Ԫ�ص���������Ϊȷ���Ͻ���ͭ����������������Ҫ�ⶨ��������__________��
(6)ʵ����ֻ�ⶨͭ����������������֤NO�IJ���������ͭ���Ͻ������ᷴӦ��ֻ���ʵ������Ϳ��ԴﵽĿ�ģ������ʵ�����________________________��
��(1)�ž�װ���еĿ��� (2)�������ɵ�����
(3)���ֺ���ɫ �� (4)��K1��K2������һ��ʱ���N2��O2
(5) ��0.50mol ��C��Բ����ƿ��ʵ��ǰ�����ӵ�����
(6)��Ӧ��Ļ��Һ�м��������NaCl��Һ�����ˣ����������õ�AgCl���������ٽ��м���
(3)���ֺ���ɫ �� (4)��K1��K2������һ��ʱ���N2��O2
(5) ��0.50mol ��C��Բ����ƿ��ʵ��ǰ�����ӵ�����
(6)��Ӧ��Ļ��Һ�м��������NaCl��Һ�����ˣ����������õ�AgCl���������ٽ��м���
��

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
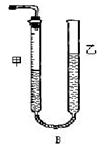
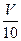 mL���Է�̪��ָʾ������c�� mol/L NaOH��Һ�ζ���ǡ����ȥV��mL��
mL���Է�̪��ָʾ������c�� mol/L NaOH��Һ�ζ���ǡ����ȥV��mL��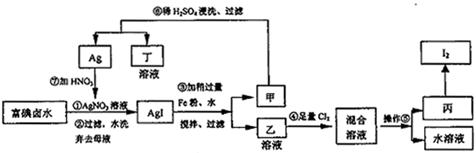
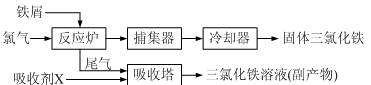
 ����ѭ�����õĸ������� ��
����ѭ�����õĸ������� ��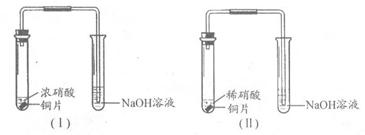
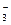 �ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������
�ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������