��Ŀ����
��8�֣���1����֪�Ͽ�1 mol H2�еĻ�ѧ��������436 kJ�������Ͽ�1 mol Cl2�еĻ�ѧ����Ҫ����243 kJ���������γ�1 molHCl�����еĻ�ѧ��Ҫ�ͷ�431 kJ����������1 mol H2��1 mol Cl2��Ӧ�������仯����H=������������
��2����֪����ʱ��0.1mol/LijһԪ��HA��ˮ����0.1%�������룬�����Һ��PH=___��___������ĵ���ƽ�ⳣ��K=___��___����HA���������H+��Ũ��ԼΪˮ���������H+��Ũ�ȵ�__��__����
��1�� -183 KJ.mol-1 ��2�� 4�� 1��10-7 ��106
���������������1����Ӧ�ȵ��ڷ�Ӧ��ļ���֮�������ļ���֮�ͣ����H=436 kJ.mol-1+243 kJ.mol-1-2��431 kJ.mol-1=-183 KJ.mol-1
��2��c(H+)=0.1mol/L��0.1%=1��10-4������pH=4����ĵ���ƽ�ⳣ��K=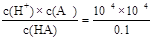 =10-7��0.1mol/L��HA��Һ����ˮ�������c(H+)Ϊ10-10��������HA���������H+��Ũ��ԼΪˮ���������H+��Ũ�ȵ�106����
=10-7��0.1mol/L��HA��Һ����ˮ�������c(H+)Ϊ10-10��������HA���������H+��Ũ��ԼΪˮ���������H+��Ũ�ȵ�106����
���㣺��Ӧ�ȣ�������ʵĵ���
�����������ۺ��Խ�ǿ�������Ѷȵͣ��Ƚϻ�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

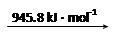 2N
(g)
2N
(g)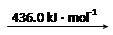 2H
(g)
2H
(g)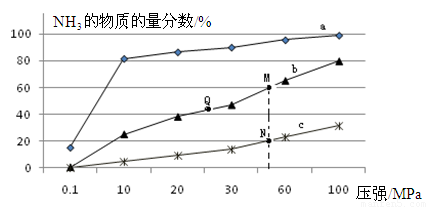
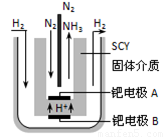 ��3������һ��DZ�ڵ������Դ������������ȼ�ϵ�ص�ȼ�ϡ���ص��ܷ�ӦΪ��
��3������һ��DZ�ڵ������Դ������������ȼ�ϵ�ص�ȼ�ϡ���ص��ܷ�ӦΪ��