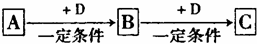��Ŀ����
1����1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��2KNO3+3C+S$\frac{\underline{\;��ȼ\;}}{\;}$A+N2��+3CO2��������ƽ���ٳ�S�⣬����Ԫ�صĵ縺�ԴӴ�С����ΪO��N��C��K��
�����������У�A�ľ�������Ϊ���Ӿ��壬�����Թ��ۼ��ķ��ӵ�����ԭ�ӹ���ӻ�����Ϊsp��
����֪CN-��N2�ṹ���ƣ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ1��1��
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2��T�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�Ϊ3d84s2��Q2+��δ�ɶԵ�������4��
��3����CrCl3��ˮ��Һ�У�һ�������´������Ϊ[CrCln��H2O��6-n]x+��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��[CrCln��H2O��6-n]x++xR-H��Rx[CrCln��H2O��6-n]+xH+
����������H+���к͵ζ����������x��n��ȷ�������ӵ���ɣ�����0.0015mol[CrCln��H2O��6-n]x+����Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200mol•L-1NaOH��Һ25.00ml����֪�������ӵĻ�ѧʽΪ[CrCl��H2O��5]2+��
���� ��1���ٷǽ�����Խǿ����縺��Խǿ��
�����������غ㶨�����жϳ�A�Ļ�ѧʽ��Ȼ���жϾ������ͣ������Թ��ۼ��ķ���ΪCO2�����ݼ۲���Ӷ����жϣ�
�۵ȵ�����Ľṹ��ͬ�����ݵ����Ľṹ������
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵڢ��壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ��ٽ�Ϻ�������Ų����ɽ��
��3�����к����ɵ�H+��Ҫ��NaOH��Һ���ɵó�H+���ʵ��������������x���ٽ��Cr�Ļ��ϼ�+3�ۣ���n��
��� �⣺��1���ٷǽ�����Խǿ����縺��Խǿ���ǽ����ԣ�O��N��C��K����縺��O��N��C��K��
�ʴ�Ϊ��O��N��C��K��
���ɻ�ѧ����ʽΪS+2KNO3+3C��A+N2��+3CO2�������������غ㶨�ɿ�֪����Ӧǰ��Ԫ�����ࡢԭ�Ӹ�����ȣ���A�Ļ�ѧʽΪK2S���������Ӿ��壻�����Թ��ۼ��ķ���ΪCO2��������̼�ĽṹʽΪO=C=O������2���۲���Ӷԣ�����Cԭ��Ϊsp�ӻ���
�ʴ�Ϊ�����Ӿ��壻sp��
��CN-��N2�ṹ���ƣ�Cԭ����Nԭ��֮���γ���������HCN���ӽṹʽΪH-C��N�������к���1���Ҽ���2���м����������ڦҼ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ1��1��
�ʴ�Ϊ��1��1��
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵڢ��壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ�NiԪ����28��Ԫ�أ�Niԭ�Ӽ۵����Ų�ʽΪ3d84s2��Fe2+�ĺ�������Ų�ʽΪ1s24s22p63s23d6��3d�ܼ���4�������ӣ�
�ʴ�Ϊ��3d84s2��4��
��3��[CrCl��H2O��5]2+��2�֣��к����ɵ�H+��Ũ��Ϊ0.1200mol/L����������Һ25.00mL������Եó�H+�����ʵ���Ϊ0.12mol/L��25.00��10-3L=0.0030mol������x=$\frac{0.0030}{0.0015}$=2��Cr�Ļ��ϼ�Ϊ+3�ۣ����[CrCln��H20��6-n]x+����3-n=2�����Ե�֪n=1�����������ӵĻ�ѧʽΪ[CrCl��H2O��5]2+��
�ʴ�Ϊ��[CrCl��H2O��5]2+��
���� ���⿼���˵縺�ԡ��������͵��жϡ��ӻ�������ȵ����塢��������Ų��������ȣ�ע����յȵ�����ԭ����ע����ݡ����ڱ��мȴ���ͬһ������λ��ͬһ�塱ȷ��Ԫ�أ���Ŀ�Ѷ��еȣ�

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д����������м������ϡH2SO4�����ˣ�
������Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��������MnSO4���Ϻ�ɫ��ʧ�����ˣ�
����Ũ�����ᾧ�����룬�õ���Ʒ��
��1��H2S04�ܽ�A1203�����ӷ���ʽ��Al2O3+6H+=2Al3++3H2O
��2��MnO4- ����Fe2+�����ӷ���ʽ����������1MnO4-+5Fe2++8H+=1Mn2++5Fe3++4H2O
��3����֪�������������������pH
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�ģ�pHԼΪ3ʱ��Fe2+��Al3+�����γɳ�������Fe2+����ΪFe3+����ʹ����ȫ����
��4����֪��һ�������£�MnO4- ����Mn2+��Ӧ����MnO2��
�����ij����м���ŨHCI�����ȣ���˵�������д���MnO2�����������ɻ���ɫ���壮
�ڢ��м���MnS04��Ŀ���dz�ȥ������MnO4-��
| A�� | 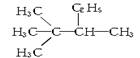 �� ��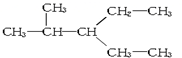 | B�� | 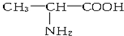 ��CH3-CH2-CH2-NO2 ��CH3-CH2-CH2-NO2 | ||
| C�� | CH3COOCH2CH3��CH3CH2COOH | D�� | C2H5-O-C2H5��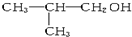 |
| A�� | �ɼױ��Ƽ������顢�������������� | |
| B�� | ��ϩʹ��ˮ��ɫ����Ȳʹ���Ը������ˮ��Һ��ɫ | |
| C�� | ����ϩ�ƾ���ϩ��������ϩ�ƾ�����ϩ | |
| D�� | �ɱ���������������������ȡ�Ҵ� |
| A�� | v��NH3��=0.6 mol•L-1•min-1 | B�� | v��N2��=0.005 mol•L-1•s-1 | ||
| C�� | v��H2��=0.9 mol•L-1•min-1 | D�� | v��NH3��=0.02 mol•L-1•s-1 |
| A�� | O2��O3 | B�� | ${\;}_{1}^{1}$H��${\;}_{1}^{2}$H | ||
| C�� | CH3CH3��CH3CH2 CH3 | D�� | CH3CH2CH2CH3��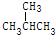 |