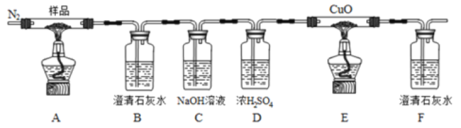
ЬтФПФкШн
ЁОЬтФПЁПЙЄвЕЩЯвдФГШэУЬПѓ(жївЊГЩЗжЮЊMnO2ЃЌЛЙКЌгаSiO2ЁЂAl2O3ЕШдгжЪ)ЮЊдСЯЃЌРћгУбЬЕРЦјжаЕФSO2жЦБИMnSO4ЁЄH2OЕФСїГЬШчЯТЃК
ЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
A. ТЫдќAЕФжївЊГЩЗжФмЕМЕчЃЌПЩжЦБИЙтЕМЯЫЮЌ
B. ЁАЫсНўЁБжївЊЗДгІЕФРызгЗНГЬЪНЮЊMnO2+SO2=Mn2++SO42-
C. МгАБЫЎЕїНкpHЕФФПЕФЪЧГ§Al3+
D. ВйзїIЮЊНсОЇЁЂЙ§ТЫЁЂЯДЕгЁЂИЩдя
ЁОД№АИЁПA
ЁОНтЮіЁП
ШэУЬПѓжївЊГЩЗжЮЊMnO2ЃЌЛЙКЌгаSiO2ЁЂAl2O3ЕШдгжЪЃЌМгЯЁСђЫсдйЭЈШыSO2КѓЃЌЙ§ТЫКѓТЫдќAЮЊSiO2ЃЌТЫвКЮЊAl3+ЁЂMn2+ЁЂSO42-МгШыАБЫЎКѓЙ§ТЫЃЌТЫдќBЮЊAl(OH)3,ТЫвККЌгаMn2+ЁЂSO42-,ОЙ§МгШШХЈЫѕЃЌРфШДНсОЇМДПЩЕФЕНMnSO4![]() H2OЁЃ
H2OЁЃ
A. ТЫдќAЮЊSiO2ЃЌВЛФмЕМЕчЃЌПЩжЦБИЙтЕМЯЫЮЌЃЌЙЪAДэЮѓЃЛB. ЁАЫсНўЁБжаMnO2гаЧПбѕЛЏадЃЌSO2ОпгаЛЙдадЃЌСНепжївЊЗДгІЕФРызгЗНГЬЪНЮЊMnO2+SO2=Mn2++SO42-ЃЌЙЪBе§ШЗЃЛC. МгАБЫЎЕїНкpHЕФФПЕФЪЧЃКAl3++3OH-=Al(OH)3![]() ,Г§ШЅAl3+ЃЌЙЪCе§ШЗЃЛD. ОЙ§НсОЇЁЂЙ§ТЫЁЂЯДЕгЁЂИЩдяВйзїПЩвдЕУЕНMnSO4ЁЄH2OЃЌЙЪDе§ШЗЃЛД№АИЃКAЁЃ
,Г§ШЅAl3+ЃЌЙЪCе§ШЗЃЛD. ОЙ§НсОЇЁЂЙ§ТЫЁЂЯДЕгЁЂИЩдяВйзїПЩвдЕУЕНMnSO4ЁЄH2OЃЌЙЪDе§ШЗЃЛД№АИЃКAЁЃ

 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
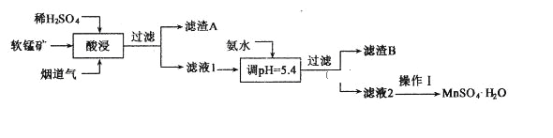
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИЁОЬтФПЁПH2C2O4ЮЊЖўдЊШѕЫсЃЌЧвОпгаЛЙдадЁЃ
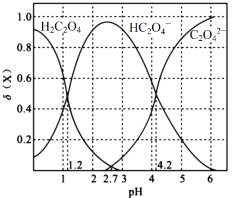
ЂёЃЎ20ЁцЪБЃЌИФБф0.1molЁЄLЃ1H2C2O4ШмвКЕФpHЃЌШмвКжаЕФH2C2O4ЁЂHC2O4ЁЊЁЂC2O42ЁЊЕФЮяжЪЕФСПЗжЪ§ІФ(X)ЫцpHЕФБфЛЏШчЭМЫљЪОЁЃ
вбжЊ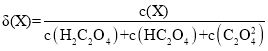
ЃЈ1ЃЉKa1(H2C2O4)ЃН___________ЁЃ
ЃЈ2ЃЉ0.1molЁЄLЃ1NaHC2O4ШмвКжаРызгХЈЖШгЩДѓЕНаЁЕФЫГађЪЧ___________ЁЃ
ЃЈ3ЃЉвбжЊ20ЁцЪБK(HCOOH)=1.77ЁС10-4ЃЌЯђHCOONaШмвКжаМгШыЩйСПH2C2O4ЃЌЗДгІЕФРызгЗНГЬЪНЪЧ___________ЁЃ
ЂђЃЎKMnO4ШмвКГЃгУзїбѕЛЏЛЙдЗДгІЕЮЖЈЕФБъзМвКЃЌгЩгкKMnO4ЕФЧПбѕЛЏадЃЌЫќЕФШмвККмШнвзБЛПеЦјжаЛђЫЎжаЕФФГаЉЩйСПЛЙдадЮяжЪЛЙдЃЌвђДЫЪЙгУЧАаыгУH2C2O4ЁЄ2H2OХфжЦЕФБъзМШмвКБъЖЈЃЈвбжЊЃК5H2C2O4+2KMnO4+3H2SO4=10CO2Ёќ+2MnSO4+K2SO4+8H2OЃЉЁЃ
ЃЈ4ЃЉзМШЗСПШЁвЛЖЈЬхЛ§ЕФKMnO4ШмвКашвЊЪЙгУЕФвЧЦїЪЧ____________ЁЃ
ЃЈ5ЃЉФГбЇЩњИљОн3ДЮЪЕбщЗжБ№МЧТМгаЙиЪ§ОнШчБэЫљЪОЃЌИУKMnO4ШмвКЕФЮяжЪЕФСПХЈЖШЮЊ___________molЁЄLЃ1ЁЃ
ЪЕбщађКХ | ЯћКФ0.1000molЁЄLЃ1ЕФH2C2O4ШмвКЕФЬхЛ§/mL | Д§ВтKMnO4ШмвКЕФЬхЛ§/mL |
1 | 29.90 | 25.00 |
2 | 30.00 | 25.00 |
3 | 30.10 | 25.00 |
ЃЈ6ЃЉдкЩЯЪіЕЮЖЈЙ§ГЬжаЃЌЯТЪіВйзїПЩЕМжТБЛВтЖЈЕФKMnO4ХЈЖШЦЋИпЕФЪЧ___________
aЃЎЮДгУБъзМвКШѓЯДЕЮЖЈЙм
bЃЎДяЕНЕЮЖЈжеЕуЃЌЖСЪ§ЪБИЉЪгвКУц
cЃЎЪЂзАД§ВтвКЕФзЖаЮЦПгУеєСѓЫЎЯДЙ§ЃЌЮДШѓЯД
dЃЎЕЮЖЈЧАЕЮЖЈЙмЯТЖЫМтзьжагаЦјХнЃЌЕЮЖЈКѓЦјХнЯћЪЇ