��Ŀ����
����Ŀ��PEI[![]() ]��һ�ַǽᾧ�����ϡ���ϳ�·�����£�ijЩ��Ӧ�������Լ�����ȥ������֪��
]��һ�ַǽᾧ�����ϡ���ϳ�·�����£�ijЩ��Ӧ�������Լ�����ȥ������֪��

i. ![]()
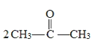
ii.CH3COOH + CH3COOH
+ CH3COOH
��1��AΪ��״����A�Ļ�ѧ����Ϊ______��
��2��A��B�ķ�Ӧ����Ϊ______��
��3�����й���D��˵������ȷ����______������ĸ����
a��������̼̼˫�� b�������ۺ���ĵ��� c������������ˮ����
��4��F��4-��-1��2-���ױ��������Ƶá�F�����������У�Cl��______��
��5��C�ĺ˴Ź��������У�ֻ��һ�����շ塣����2-�����Ϊ�л�ԭ�ϣ�ѡ�ñ�Ҫ�����Լ�Ҳ�ܺϳ�C��д���йػ�ѧ����ʽ��_____
��6����E��KΪԭ�Ϻϳ�PEI��Ϊ������Ӧ��
![]()
д���м����2�Ľṹ��ʽ�� _______
���𰸡���ϩ �ӳɷ�Ӧ ab ��COOH ![]() +NaOH
+NaOH![]()
![]() +NaBr��
+NaBr��![]() +O2
+O2![]()
 +2H2O
+2H2O 
��������
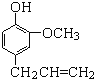
��������Ϣ��֪��AΪ��״������Aֻ��Ϊ��ϩ���뱽����һ�������·�Ӧ�����ɱ�������BΪ����������������Ӧ�����ݷ���ʽ����֪CΪ��ͪ��DΪ���ӣ���ͪ�뱽���ڴ��������»�����E��![]() ����������ϳɷ�������֪������PEI[
����������ϳɷ�������֪������PEI[![]() ]�ĵ��壬�ٽ�����⣬����֪4-��-1��2-�����������Ʊ�F����FΪ
]�ĵ��壬�ٽ�����⣬����֪4-��-1��2-�����������Ʊ�F����FΪ �����ݸ�����Ϣii��֪��KΪ
�����ݸ�����Ϣii��֪��KΪ ���ݴ˷�������
���ݴ˷�������
��1��AΪ��״�������ݷ��ӿ����жϣ�Aֻ���DZ�ϩ��
��2��A��BΪ��ϩ�뱽��Ӧ���ɱ����Ĺ��̣��䷴Ӧ����Ϊ�ӳɷ�Ӧ��
��3��������������ԭ����֪�����ӳ�������ˮ�е��ܽ�Ȳ���������в�����̼̼˫�����������ȩ�������۷�Ӧ���ʴ�Ϊ��ab��
��4��4-��-1��2-���ױ��������õ�F����F�Ľṹ��ʽΪ ��������������������У�Cl����COOH���ʴ�Ϊ����COOH��
��������������������У�Cl����COOH���ʴ�Ϊ����COOH��
��5������2-�����Ϊ�л�ԭ�ϣ�������������ˮ��Һ��ˮ������2-������Ȼ��2-�������������ɱ�ͪ���йط�Ӧ�Ļ�ѧ����ʽΪ![]() ��
��![]() ��
��
��6����E��K�Ľṹ��������Ϣ![]() ��֪���м����1Ϊ
��֪���м����1Ϊ �������м����1�Ľṹ����Ϣ
�������м����1�Ľṹ����Ϣ ��֪���м����2�Ľṹ��ʽΪ
��֪���м����2�Ľṹ��ʽΪ ��
��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ����ɽ�д��조ɽˮ�ֳ��С�������о�NOx��SO2�ȴ�����Ⱦ������ƴ���������Ҫ���塣
��1��SO2���ŷ���Ҫ������ú��ȼ�գ���ҵ�ϳ��ð�ˮ���շ�����β���е�SO2����֪���չ�������ط�Ӧ���Ȼ�ѧ����ʽ���£�
SO2(g)+NH3��H2O(aq)![]() NH4HSO3(aq) ��H1=a kJ��mol1��
NH4HSO3(aq) ��H1=a kJ��mol1��
NH3��H2O(aq)+ NH4HSO3(aq)![]() (NH4)2SO3(ag)+H2O(l) ��H 2=b kJ��mol1��
(NH4)2SO3(ag)+H2O(l) ��H 2=b kJ��mol1��
2(NH4)2SO3(aq)+O2(g)![]() 2(NH4)2SO4(aq) ��H 3=c kJ��mol1��
2(NH4)2SO4(aq) ��H 3=c kJ��mol1��
��Ӧ2SO2(g)+4NH3��H2O(aq)+O2(g) ![]() 2(NH4)2SO4(aq)+2H2O(l)�Ħ�H =____kJ��mol1��
2(NH4)2SO4(aq)+2H2O(l)�Ħ�H =____kJ��mol1��
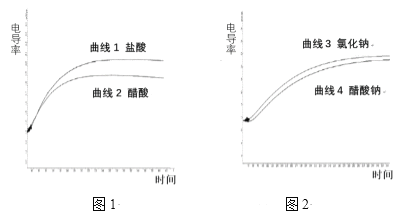
��2������ϩ(C2H4)��Ϊ��ԭ������(NO)������������ͼ1�����ܷ�Ӧ�Ļ�ѧ����ʽΪ_______�����������¶ȡ�������(����ɸ�д�������������)�Ĺ�ϵ��ͼ2��Ϊ�ﵽ�������Ч����Ӧ���õ�������______��

��3��T1�¶�ʱ���ݻ�Ϊ2L�ĺ����ܱ������з�����Ӧ��2NO(g)+O2(g)![]() 2NO2(g) ��H<0��ʵ���ã�����=��(NO)����=2��(O2)����=k��c2(NO)��c(O2)������=(NO2) ����=k��c2(NO2)��k����k��Ϊ���ʳ���ֻ���¶�Ӱ�졣��ͬʱ�̲��������n(NO)��n(O2)�����
2NO2(g) ��H<0��ʵ���ã�����=��(NO)����=2��(O2)����=k��c2(NO)��c(O2)������=(NO2) ����=k��c2(NO2)��k����k��Ϊ���ʳ���ֻ���¶�Ӱ�졣��ͬʱ�̲��������n(NO)��n(O2)�����
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 1 | 0.6 | 0.4 | 0.2 | 0.2 | 0.2 |
n(O2)/mol | 0.6 | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 |
��T1�¶�ʱk��/k��=__________ L/mol��
�������������¶ȸı�ΪT2ʱ��k��=k������T2__________T1(�>������<����=")��
��4����֪��N2(g)+O2(g)![]() 2NO(g) ��H��+181.5 kJ��mol��1��ij����С�鳢�����ù����������ս���NO�ķֽ⡣����
2NO(g) ��H��+181.5 kJ��mol��1��ij����С�鳢�����ù����������ս���NO�ķֽ⡣����![]() ��
��![]() ��
��![]() ��
��![]() �ֱ��ʾN2��NO��O2����������ڹ����������ֽ�NO�Ĺ�����ͼ��ʾ���������������Ĺ����У�����״̬��͵���___(����ĸ���)��
�ֱ��ʾN2��NO��O2����������ڹ����������ֽ�NO�Ĺ�����ͼ��ʾ���������������Ĺ����У�����״̬��͵���___(����ĸ���)��

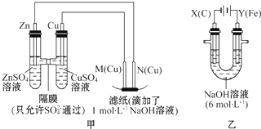
��5�����õ�ⷨ�������¿�����ϡ����NO(O2Ũ��ԼΪNOŨ�ȵ�10��)��װ��ʾ��ͼ���£��������ʿɴ���O2��

�������ĵ缫��ӦʽΪ______��
������һ������NO�����ĵĵ���ԶԶ�������ۼ����������ܵ�ԭ����(��������������)_________��