��Ŀ����
����Ŀ���ࣨGe���ǵ��͵İ뵼��Ԫ�أ��ڵ��ӡ����ϵ�����Ӧ�ù㷺���ش��������⣺
��1����̬Geԭ�ӵĺ�������Ų�ʽΪ[Ar]____________����__________��δ�ɶԵ��ӡ�
��2��Ge��C��ͬ��Ԫ�أ�Cԭ��֮������γ�˫������������Geԭ��֮�������γ�˫������������ԭ�ӽṹ�Ƕȷ�����ԭ����________________��
��3���Ƚ�������±������۵�ͷе㣬������仯���ɼ�ԭ��_____________________��
GeCl4 | GeBr4 | GeI4 | |
�۵�/�� | 49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
��4�������ԭCO2�Ʊ�CH4��Ӧ�У���״����Zn2GeO4�Ǹ÷�Ӧ�����ô�����Zn��Ge��O�縺���ɴ���С��˳����______________��

��5��Ge�������н��ʯ�ͽṹ������Geԭ�ӵ��ӻ���ʽΪ______________��֮����ڵ���������_____________��
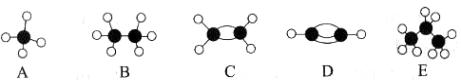
��6����������������Ҫ�أ���ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�ã���ͼΪGe�����ľ���������ԭ���������AΪ��0,0,0����BΪ��![]() ��0��
��0��![]() ����C��
����C��![]() ��
��![]() ��0������Dԭ�ӵ��������Ϊ______��
��0������Dԭ�ӵ��������Ϊ______��
�ھ������������������Ĵ�С����״����֪Ge�����ľ�������a=565.76 pm�����ܶ�Ϊ__________g��cm-3���г�����ʽ���ɣ���
���𰸡���1��3d104s24p2�� 2
��2�����ԭ�Ӱ뾶��ԭ��֮���γɵ��������ϳ���p-p����粢���ص��̶Ⱥ�С�������ص��������γɦм�
��3��GeCl4��GeBr4��GeI4�ۡ��е��������ߣ�ԭ���Ƿ��ӽṹ���ƣ���Է����������������Ӽ������������ǿ
��4��O��Ge��Zn
��5��sp3�����ۼ�
��6����(![]() ��
��![]() ��
��![]() )
)
��![]()
��������
���������
��1��Ge��32��Ԫ�أ�λ�ڵ������ڵ�IVA�壬��̬Geԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s24p2
��[Ar]3d104s24p2����������4s�ܼ���2������Ϊ�ɶԵ��ӣ�4p�����2�����ӷֱ��Բ�ͬ��
����ڣ���2���δ�ɶԵ�����
�ʴ�Ϊ��3d104s24p2��2��
��2����ȻGe��C��ͬ��Ԫ�أ�Cԭ��֮������γ�˫����������������Ge��ԭ�Ӱ뾶������ͨ��
���粢�硱��ʽ�γɦм�������Geԭ��֮�������γ�˫����������
�ʴ�Ϊ��Geԭ�Ӱ뾶��ԭ�Ӽ��γɵĦҵ����ϳ���p-p����粢���ص��̶Ⱥ�С�������ص��������γɦм���
��3�����±���ﶼ�Ƿ��Ӿ��壬���Ӽ�ͨ�����Ӽ���������ϣ����������ṹ���Ƶķ��Ӿ��壬��
�Է�������Խ���Ӽ�������Խǿ���۷е�Խ�ߣ�������Է���������GeCl4��GeBr4��GeI4����
�е㣺GeCl4��GeBr4��GeI4��
�ʴ�Ϊ��GeCl4��GeBr4��GeI4���ۡ��е�����������ԭ���Ƿ��ӽṹ���ƣ����������������Ӽ������������ǿ��
��4��Ԫ�طǽ����ԣ�Zn��Ge��O��Ԫ�صķǽ�����Խǿ���������ӵ�����Խǿ��Ԫ�صĵ縺��Խ��
�ʵ縺�ԣ�O��Ge��Zn��
�ʴ�Ϊ��O��Ge��Zn��
��5��Ge�������н��ʯ�ͽṹ��Geԭ������Χ4��Geԭ���γ���������ṹ����ռ������������
״�ṹ������ԭ�Ӿ��壬Geԭ��֮���γɹ��ۼ���Geԭ���ӻ������ĿΪ4����ȡsp3�ӻ���
�ʴ�Ϊ��sp3�����ۼ���
��6����D����Χ4��ԭ���γ���������ṹ��D�붥��A�����ߴ��ھ�����Խ����ϣ�������B��C
���ϵ�������ԭ�ӵ�ƽ����ƽ�в��潫����2�ȷ֣�ͬ����Dԭ�ӵ���ƽ������ƽ�潫�����
����2�ȷݣ���֪D���ڵ��������![]() ������Dԭ�ӵ��������Ϊ��
������Dԭ�ӵ��������Ϊ��![]() ��
��![]() ��
��![]() ����
����
�ʴ�Ϊ��![]() ��
��![]() ��
��![]() ����
����
�ھ�����Geԭ����ĿΪ4+8��![]() +6��
+6��![]() =8����ϰ���٤������������������������Ϊ
=8����ϰ���٤������������������������Ϊ
![]() ����������a=565.76pm�����ܶ�Ϊ
����������a=565.76pm�����ܶ�Ϊ![]() ��(565.76��10-10cm)3=
��(565.76��10-10cm)3=
![]() gcm-3��
gcm-3��
�ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����֪��Fe(OH)3��Al(OH)3�������������ܽ��pH���±���
�������� | ��ҺpH | |||
��ʼ���� | ������ȫ | ������ʼ�ܽ� | ������ȫ�ܽ� | |
Fe(OH)3 | 2.3 | 3.4 | ���� | ���� |
Al(OH)3 | 3.3 | 5.2 | 7.8 | 12.8 |
��FeCl3��Al2(SO4)3�Ļ����Һ����μ���Ba(OH)2��Һ���γɳ������������ͼ��ʾ�������ƶϴ������
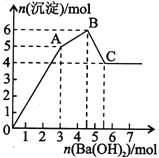
A��AB�ο��ܷ����ķ�Ӧ��2Ba2++3OH��+2SO42��+Al3+=2BaSO4��+Al(OH)3��
B��C���Ӧ�ij�����Fe(OH)3��BaSO4
C��OA�ο��ܷ����ķ�Ӧ��3Ba2++6OH��+3SO42��+Al3++Fe3+=3BaSO4��+Fe(OH)3��+Al(OH)3��
D����ͼ����ԭ��Һ�У�c(Cl��)=c(SO42��)
����Ŀ���ᴿ����������ѡ�õij������Լ�����Ҫ���뷽������ȷ���ǣ�������Ϊ�������ʣ���
�������� | �����Լ� | ���뷽�� | |
A | �����ױ��� | KMnO4���ữ����NaOH��Һ | ��Һ |
B | NH4Cl��Һ��FeCl3�� | NaOH��Һ | ���� |
C | �������������ᣩ | KOH��Һ | ��Һ |
D | �������ӣ� | ŨBr2ˮ | ���� |