��Ŀ����
��һ�ְ�ɫ��ĩ���������������Ӻ��������еļ��֡�
�����ӣ�S2����Cl����NO3����SO42����CO32����HCO3����MnO4����
�����ӣ�Na����Mg2����Al3����Ba2����Fe2����Fe3����Cu2����NH4+��
���ð�ɫ��ĩ��������ʵ�飬�۲쵽���������£�
| ʵ����� | ���� |
| a.ȡ������ĩ����ˮ���� | ȫ���ܽ⡢ |
| ��Һ��ɫ�� | |
| b.��������Һ�����������������Һ�������� | ���������� |
| c.ȡ������ĩ�������� | ���������� |
| d.ȡ������ĩ����ϡH2SO4��ϡHNO3�Ļ��Һ | �а�ɫ�������� |
����ʵ���ƶϣ�
(1)��aʵ���У����ƶϷ�ĩ�в�������______________(�����ӷ��ţ���ͬ)��
(2)��bʵ���У����ƶϷ�ĩ�в�������_____________________________________��
(3)��cʵ���У����ƶϷ�ĩ�в�������________________________________��
(4)��dʵ���У����ƶϷ�ĩ�в�������________��һ������________��
(5)���ϸ�ʵ������ȷ���Ƿ���ڵ�������____________��
(1)Fe2����Fe3����Cu2����MnO4��
(2)NH4+��Mg2����Al3��
(3)CO32����HCO3����S2��
(4)SO42����Ba2��
(5)Cl����NO3����Na��
����

��ϰ��ϵ�д�
�����Ŀ
������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH4+ |
| ������ | OH����NO3����I����HCO3����AlO2����HSO4�� |
(1)��A��B��ˮ��Һ��Ϊ��ɫ��B��ˮ��Һ�ʼ��ԣ��һ�Ϻ�ֻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������塣
��B�Ļ�ѧʽΪ__________________��
��A��B��Һ��Ϻ���ȳ����ԣ���Ӧ�����ӷ���ʽΪ__________________________��
(2)��A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��AΪ_______________��
�ھ�����������������Һ��Ƶ�ԭ����������֣�
��._______ _____________________�� ��.___________ ____________��
������һ������֤��������Һ��Ƶ�ԭ��__________________________________��
��������Һ���ԭ����������Ƴ�ԭ��أ���������a����b����b���ĵ缫��ӦʽΪ_______________________________________
���ȸʯ��һ�ֺ�ͭ�Ŀ�ʯ����ͭ��̬Ϊ
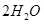 ��ͬʱ����
��ͬʱ����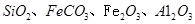 �����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
�����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��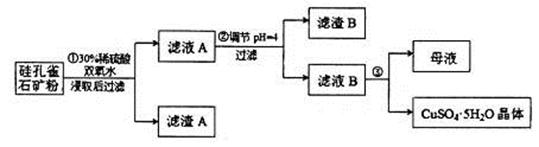
��ش��������⣺
��1����ɲ������ϡ������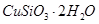 ������Ӧ�Ļ�ѧ����ʽ
������Ӧ�Ļ�ѧ����ʽ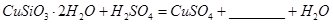 ��
��
�����ӷ���ʽ��ʾ˫��ˮ������_____________________________��
��2������ڵ�����ҺpHѡ�õ�����Լ���__________________
A��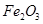 | B��CuO | C��A12O3 | D��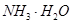 |
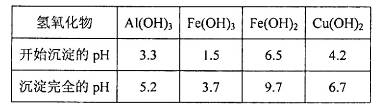
���ϱ���֪������ҺpH=4ʱ��������ȫ��ȥ��������______��������ȫ��ȥ��������________��
��4����ҺBͨ������Ũ���������Ũ��Ϊԭ����һ�룩����ȴ�ᾧ���Եõ�
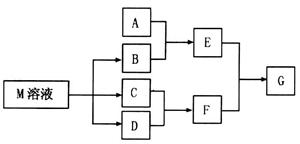
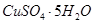 ���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�
���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�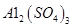 ������Һ��
������Һ�� mol
mol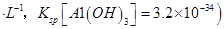 ______________��
______________����5����Ҫ�ⶨ����ͭ�����нᾧˮ�ĺ�������Ҫ�������Ǿƾ��ơ�������ƽ�����Ǽܡ������ǡ���������������������ǯ���в���ҩ�ס�_________________��ʵ�����������ͭ�������ʧˮ���ڿ�����ȴ���������ⶨ���______________(�ƫ�ߡ��� ��ƫ�͡����䡱)��
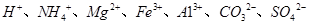 �е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��
�е�ijЩ���ӣ��������Һ�м���B��Һʱ�������ɳ��������ʵ�����B��Һ����������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е����Ӽ���Ũ��֮��Ϊ ��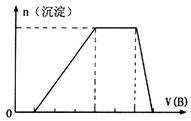

 ��ɫ����
��ɫ���� �������ܽ�
�������ܽ�