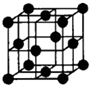
��Ŀ����
��10�֣���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ���ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�8��Ԫ�ء�D��ԭ��������EС6��D��B���γ����ӻ������侧���ṹ����ͼ����ش�
��1��A��B�γɵĻ������ڹ�̬ʱ�ľ��������� ��A��B�γɵĻ������A��C�γɵĻ������۵�Ҫ�ߣߣߣߣ���ߡ��ͣ�
��2��д��C�ĵ�����ˮ��Ӧ�����ӷ���ʽ ��
��3����ͼ��ʾ��D��B�γɵ����ӻ�����Ļ�ѧʽΪ ����������ӻ������Ƿ�Ϊ���壬��ɿ��Ŀ�ѧ������ �������ӻ����ᄃ����ܶ�Ϊag��cm-3��B��D��Ԫ�ص����ԭ�������ֱ�Ϊb��c����������� cm3��ֻҪ���г���ʽ����
����������ǰ36��Ԫ���У��������ڱ���1��18���еĵ�8��Ԫ�ص���������E��������D��Ca������D��B�γ����ӻ����ᄃ���ṹ���жϣ������ӻ�������DB2����B��F��Cl����ΪB��C��ͬһ���壬��ԭ������ǰ��С�ں��ߣ�����Bֻ����F��C��Cl��A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ�壬����A��H��
��1��HF���ڷ��Ӿ��壬����HF�к������������HF�ķе�����Ȼ���ġ�
��2����������ˮ��������ʹ����ᡣ
��3���������ӻ������Ƿ�Ϊ���壬��ɿ��Ŀ�ѧ�������������䷨���ڸþ����к��еĸ�������8��1/8��6��1/2��4����F����8�����Ծ���������ǣ�8b+4c��/a NA��
��1�����Ӿ��塡�ߣ�2��Cl2+H2O=H++Cl-+HClO
��3��CaF2 ��X�������䷨����8b+4c��/a NA��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�