��Ŀ����
7���й�Ԫ��X��Y��Z��D��E����Ϣ����| Ԫ�� | �й���Ϣ |
| X | Ԫ����Ҫ���ϼ�Ϊ-2��ԭ�Ӱ뾶Ϊ0.074nm�� |
| Y | ��������������������������֮��Ϊ4�� |
| Z | ԭ�Ӱ뾶Ϊ0.102nm����������������������Ӳ�����2�����䵥����X�ĵ�����ȼ�գ���������������ɫ���森 |
| D | ����������Ӧ��ˮ����ܵ����������������������ȵ����������ӣ� |
| E | �����������г�������������Ʒ�ڳ�ʪ�������ױ���ʴ���� |
��1��X��һ���⻯�������ʵ������ȡX�ĵ��ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��2��EԪ����YԪ�ؿ��γ�EY2��EY3���ֻ��������˵������ȷ���Ǣڢۣ�����ţ���
�ٱ���EY2��Һʱ��������Һ��������E���ʣ�
��ͨ��ʵ��������EY3��Һʱ��ֱ����ˮ�ܽ�EY3���弴�ɣ�
��EY2ֻ��ͨ���û���Ӧ���ɣ�EY3ֻ��ͨ�����Ϸ�Ӧ���ɣ�
��ͭƬ��̼����EY3��Һ���ԭ��أ�������ͭƬ�ص�������̼����
��3���û�ѧ���ű�ʾD2Z��ˮ��Һ�и�����Ũ�ȴӴ�С��˳����Na+��S2-��OH-��HS-��H+��
���� XԪ����Ҫ���ϼ�Ϊ-2������VIA�壬Z������X�ĵ�����ȼ�գ���������ɫ���棬��ZΪSԪ�ء�XΪOԪ�أ�Y��������������������������֮��Ϊ4��Y�����ڵڶ����ڣ���Ϊ��Ԫ�أ���X��ͬ�����������⣬��X���ڵ������ڣ��ʴ��ڢ�A��YΪClԪ�أ�D������������Ӧ��ˮ����ܵ����������������������ȵ����������ӣ���DΪNa��E�����������г�������������Ʒ�ڳ�ʪ�������ױ���ʴ������EΪFeԪ�أ��ݴ˽��
��� �⣺XԪ����Ҫ���ϼ�Ϊ-2������VIA�壬Z������X�ĵ�����ȼ�գ���������ɫ���棬��ZΪSԪ�ء�XΪOԪ�أ�Y��������������������������֮��Ϊ4��Y�����ڵڶ����ڣ���Ϊ��Ԫ�أ���X��ͬ�����������⣬��X���ڵ������ڣ��ʴ��ڢ�A��YΪClԪ�أ�D������������Ӧ��ˮ����ܵ����������������������ȵ����������ӣ���DΪNa��E�����������г�������������Ʒ�ڳ�ʪ�������ױ���ʴ������EΪFeԪ�أ�
��1��X��һ���⻯�������ʵ������ȡX�ĵ��ʣ�Ӧ�ǹ�������ֽ�����ˮ���������䷴Ӧ�Ļ�ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
�ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��2��EԪ����YԪ�ؿ��γ�FeCl2��FeCl3���ֻ����
���������������ױ�����������FeCl2��Һʱ��������Һ��������Fe���ʣ�����ȷ��
����������ˮ�⣬ͨ��ʵ��������FeCl3��Һʱ�����Ȼ����ܽ��������У��ʴ���
��FeCl2�������Ȼ�����Fe��Ӧ�õ������ڻ��Ϸ�Ӧ���ʴ���
��ͭƬ��̼����FeCl3��Һ���ԭ��أ�CuΪ������̼��Ϊ������������ͭƬ�ص�������̼��������ȷ��
��ѡ���ڢۣ�
��3��Na2S��ˮ��Һ��S2-ˮ�⣬��Һ�ʼ��ԣ���Һ������������Դ��ˮ�ĵ��롢S2-��HS-ˮ�⣬����Һ�и�����Ũ�ȴӴ�С��˳���ǣ�Na+��S2-��OH-��HS-��H+��
�ʴ�Ϊ��Na+��S2-��OH-��HS-��H+��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ѶȲ����ƶ�Ԫ���ǽ���ؼ���ע��Ի���֪ʶ���������գ�

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�| A�� | ���ʵ������߸�����������֮һ������Ϊmol | |
| B�� | Ħ����������ֵ�ϵ��ڸ����ʵ���Է���������������ԭ������ | |
| C�� | ��״���£������Ħ�����Ϊ22.4L | |
| D�� | 11.7���Ȼ�������1000����ˮ�У��������ʵ����ʵ���Ũ��Ϊ0.2mol•L-1 |
| A�� | +1 | B�� | +2 | C�� | +3 | D�� | +4 |
��1����⾫��ͭʱ��ͭ���������������缫��ӦʽΪCu2++2e-�TCu���������У����Һ��c��Cu2+����С������С�����䣩���Ե���Һ�������´���
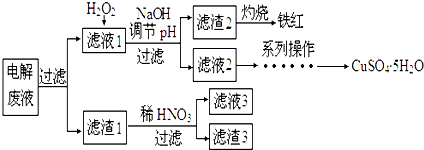
��֪����ijЩ���������γ��������������pH���
| ������ | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Zn��OH��2 |
| ��ʼ���� | 2.3 | 7.5 | 4.7 | 5.4 |
| ��ȫ���� | 3.2 | 9.7 | 6.7 | 8.0 |
��2����Һl�м���H2O2������Ӧ�����ӷ���ʽ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��3����NaOH��Һ������ҺpH�ķ�Χ��3.2��pH��4.7��
��4������1��ϡHNO3��Ӧ�Ļ�ѧ����ʽ��3Ag+4HNO3�T3AgNO3+NO��+2H2O��
| A�� | 2mol/L | B�� | 1mol/L | C�� | 0.1mol/L | D�� | 0.05mol/L |
| A�� | ��BF3��NaCl3�����У�����ԭ�Ӷ�����8�����ȶ����� | |
| B�� | P4��CH4�ռ�ṹ��ͬ������еĻ�ѧ������Ҳ��ȫ��ͬ | |
| C�� | Cl-�Ľṹʾ��ͼ�ɱ�ʾΪ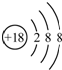 | |
| D�� | COS�ĵ���ʽ�ɱ�ʾΪ |
 ��
�� ��
�� ��
�� ����ˮú���ϳɼ״���úҺ����һ����Ҫ�������䷴Ӧ���Ȼ�ѧ����ʽΪCO��g��+2H2��g��?CH3OH��l����H=-90.8kJ•mol-1�����ݻ�Ϊ1L�ĺ����ܱ������У�����һ����CO��H2�����CO��H2�����ʵ����ı仯������ͼ��ʾ��
����ˮú���ϳɼ״���úҺ����һ����Ҫ�������䷴Ӧ���Ȼ�ѧ����ʽΪCO��g��+2H2��g��?CH3OH��l����H=-90.8kJ•mol-1�����ݻ�Ϊ1L�ĺ����ܱ������У�����һ����CO��H2�����CO��H2�����ʵ����ı仯������ͼ��ʾ��