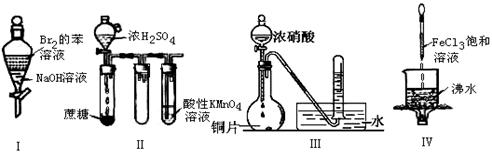
��Ŀ����
(12��)ʵ���ã�ij�л�����Է�������Ϊ72��
��1�������л��������������ʽΪ_________������______��ͬ���칹�塣��������Cl2����ȡ����Ӧ�����ɵ�һ�ȴ���ֻ��1�֣�������Ľṹ��ʽΪ_____________��
��2�������л���3.6g��ȫȼ������0.15molCO2��0.1molH2O������л���ķ���ʽΪ_______��������һ�ֲ��������ᣬ������״�����������Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ��������________ ��
������һ�ֲ������������������Ӿ۷�Ӧ��������Ľṹ��ʽΪ_______________��
��1�������л��������������ʽΪ_________������______��ͬ���칹�塣��������Cl2����ȡ����Ӧ�����ɵ�һ�ȴ���ֻ��1�֣�������Ľṹ��ʽΪ_____________��
��2�������л���3.6g��ȫȼ������0.15molCO2��0.1molH2O������л���ķ���ʽΪ_______��������һ�ֲ��������ᣬ������״�����������Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ��������________ ��
������һ�ֲ������������������Ӿ۷�Ӧ��������Ľṹ��ʽΪ_______________��
(1) C5H12 �� 3 ��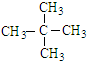
��2��C3H4O2 ��
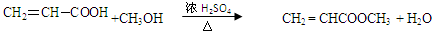 ��
��
-[-CH2-CH-]-n
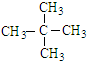
��2��C3H4O2 ��
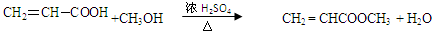 ��
��-[-CH2-CH-]-n
������Ϊ72����ΪC5H12�������顢�����顢�����顣һ�ȴ���һ�ֵ�Ϊ�����顣�����л���3.6g��ȫȼ������0.15molCO2��0.1molH2O������л���ķ���ʽΪC3H4O2 ��������һ�ֲ�����������ϩ��ͼ״�������Ӧ�õ�CH2 = CHCOOCH3

��ϰ��ϵ�д�
�����Ŀ