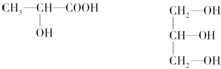��Ŀ����
3���ִ������벻�����������������������ܣ�����ڸ������м���㷺����;����1���ڸ����£���������ĩ�������ȷ�Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3��
��2��ijѧ�����������Ʊ�����Al��OH��3���Ʊ���������ͼ��ʾ��
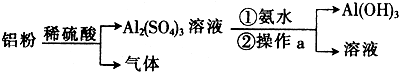
��ش�
��ͨ������a�����Al��OH��3������a�������ǹ��ˣ�
�ڴ�Al��OH��3�����ʿ��ǣ���Al2��SO4��3��Һ�Ʊ�Al��OH��3ʱ�����ð�ˮ������NaOH��Һ������һ����ѧ����ʽ���������ɣ�Al��OH��3+NaOH=NaAlO2+2H2O��
��3�������Ժ��ռ���Һ��Ӧ���÷�Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��4����ҵ��ұ���Ļ�ѧ����ʽΪ2Al2O3 $\frac{\underline{\;���\;}}{\;}$4Al+3O2����������2.7t������ת�Ƶ��ӵ����ʵ���Ϊ3��105mol��
���� ��1�����ݡ����ȷ�Ӧ���ĸ�����д��ѧ����ʽ��
��2���ٷ���������Һ�ù��˵ķ�����
���Ʊ�Al��OH��3�ð�ˮ������������������ΪAl��OH��3��������������������������ڼ
��3�������Ժ��ռ���Һ��Ӧ���÷�Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4����ҵ��ұ�����õ���������ķ�������������1molAlת��3mol���Ӽ��㣮
��� �⣺��1�������ȷ�Ӧ�����ͽ���������Ӧ�õ�����������������ѧ����ʽΪ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3���ʴ�Ϊ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3��
��2�����������мӰ�ˮ������Ӧ����������������������泥��ʽ�������������������˼��ɣ�
�ʴ�Ϊ�����ˣ�
���Ʊ�Al��OH��3�ð�ˮ�������������ƣ�����ΪAl��OH��3���������������������������ǿ�������������ƣ�
��Al��OH��3���ܽ⣺Al��OH��3+NaOH=NaAlO2+2H2O��
�ʴ�Ϊ��Al��OH��3+NaOH=NaAlO2+2H2O��
��3�������Ժ��ռ���Һ��Ӧ���÷�Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4����ҵ��ұ�����õ���������ķ�����2Al2O3 $\frac{\underline{\;���\;}}{\;}$4Al+3O2����
�˷�Ӧת��12mol����ʱ����4mol������ÿ����1molAlת��3mol���ӣ�
������2.7t��ʱת�Ƶ���xmol��
���ݣ�Al��3e-
1mol�������� 3mol����������������������������������������������������������������
2.7��106g��27g/mol x mol
���x�T3��105mol��
�ʴ�Ϊ��2Al2O3 $\frac{\underline{\;���\;}}{\;}$4Al+3O2����3��105mol��
���� �����ۺϿ��������ȷ�Ӧ�������������Ʊ������ʣ�������������ұ�����ۺ��Խ�ǿ���Ѷ����У�

 ���ӵ����������У�������ǣ�������
���ӵ����������У�������ǣ�������| A�� | �뱽��ֱ��������ԭ�Ӷ���ͬһƽ���� | |
| B�� | �����е�̼ԭ�ӷֱ��ȡsp��sp2��sp3�ӻ��������й���10��������̼ԭ�� | |
| C�� | �����18��ԭ�ӿ��ܴ���ͬһ��ƽ���� | |
| D�� | ����ʽΪC11H6ClF3 |
| A�� | �ȵĴ�����Һ����ȥ���;��ϵ����� | |
| B�� | �������ƿ�����������ߵĹ����� | |
| C�� | ��ҵ���õ�ⱥ��ʳ��ˮ��ý����� | |
| D�� | ����������ҽ�õ�θ���кͼ���һ�� |
| A�� | ������̼������Һ��Ӧ��2H++CO32-�TCO2��+H2O | |
| B�� | �Ҵ����Ʒ�Ӧ��2CH3CH2O-+2H++2Na��2CH3CH2ONa+H2�� | |
| C�� | ��������Һ��ͨ�������̼��2C6H5O-+CO2+H2O��2C6H5OH+CO32- | |
| D�� | ������Һ�м�����ˮ�� +3B2�� +3B2��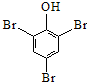 ��+3H++3Br- ��+3H++3Br- |
| A�� | �����һ�ȼ��� | B�� | ����ϩ��������ϩ | ||
| C�� | �Ҵ�����ȩ | D�� | �Ա������ᣬ�Ҷ�����������ά |
| A�� | �٢� | B�� | �ڢۢܢݢ� | C�� | �ڢ� | D�� | �ڢۢ� |
| A�� | Fe | B�� | Al��OH��3 | C�� | NO | D�� | H2SO3 |
| A�� |  | B�� |  | C�� | CH2ClCHO | D�� | HOCH2CH2OH |
 �������Ų�ʽ1s22s22p63s23p63d14s2����Ԫ�ص�ԭ������Ϊ21����Ԫ���ǽ���Ԫ�أ���������ǽ����������γɵĵ���Ϊ�������壮
�������Ų�ʽ1s22s22p63s23p63d14s2����Ԫ�ص�ԭ������Ϊ21����Ԫ���ǽ���Ԫ�أ���������ǽ����������γɵĵ���Ϊ�������壮