��Ŀ����
����Ŀ����ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ�����Һ������ɫ����ɫ������֡���һ��ʱ����־������ữ�ĸ��������Һ��ɫ��

��ͬѧ��Ϊ���Ը��������Һ��ɫ����֤����ϩ�����Ը��������Һ�����ˣ���ͬѧ��Ϊ���Ը��������Һ��ɫ������֤����ϩ�����Ը��������Һ�����ˡ�
��1������Ϊ�ĸ�ͬѧ�Ĺ۵���ȷ�� _____����ס����ҡ�����������_____ ������ţ���
A.(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ������������Ӧ
B.(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ�����˼ӳɷ�Ӧ
C.(��ƿ�����Ը��������Һ��ɫ������֤��ͨ��������Ǵ�����
D.(��)ƿ�����Ը��������Һ��ɫ��ֻ��֤��ͨ�������һ�����л�ԭ��
��2����ͬѧȡ����ƿ��������Һ���Թ������������Ȼ�����Һ��������ɫ����������Ϊ��ϩ��һ�����ж�����������Ϊ���Ľ����Ƿ�ɿ��� _____����ɿ������ɿ�������������_____________________��������Ϊ���ɿ����Ľ�����ʵ�鷽����֤����ϩ���Ƿ���SO2��__________________ ��
��3����ͬѧ������ʵ�鷽���������ʵ��Ľ�������֤����ϩ�ܷ����ӳɷ�Ӧ�����ĸĽ�������������װ�ã��ͣ���֮������һ��װ������_____��ϴ��ƿ�ҽ�����ƿ����Һ����_____��������Ӧ�Ļ�ѧ����ʽΪ_________________��
���𰸡��� C��D ���ɿ� ���Ը��������Һ�п��ܺ���SO42�� ƿ����Һ����Ʒ����Һ NaOH��Һ ������Ȼ�̼��Һ 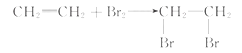
��������
(1)Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ���۲쵽��ƿ����Һ��ڣ�˵��Ũ���������Ҵ�����̼��ͬʱ��������ԭ���Ƶõ���ϩ������ͨ���Ậ��CO2��SO2�����壬���������������ܷ���������ԭ��Ӧ�����������Һ��ɫ��
(2)�����ữ�ĸ��������Һ�б����ͺ���SO42-��
(3)����������Һ�ɳ�����������������Ȼ�̼��Һ��ɫ��֤����ϩ�ܷ����ӳɷ�Ӧ��
(1)Ũ�������ǿ�����ԣ��������Ҵ������Ҵ�������̼��ͬʱ��������ԭ�ɶ��������������������ط�Ӧ5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4��ɫ����ϩ�����������Һ����5CH2=CH2+12 KMnO4+18 H2SO4 ��10CO2+12 MnSO4+28 H2O +6 K2SO4�����¸��������Һ��ɫ��
A.(��)ƿ�����Ը��������Һ��ɫ�������Ƕ��������������ط�Ӧ����A����
B.(��)ƿ�����Ը��������Һ��ɫ����������ϩ�����������������B����
C. ��ʹ���������ɫ���ж����������ϩ�����Բ���֤��ͨ��������Ǵ��������Cѡ������ȷ�ģ�
D. �����Ƕ�����������ϩ��ʹ���������ɫ�������������ط���������ԭ��Ӧ���Ҹ���������������������ǻ�ԭ�����л�ԭ�ԣ�����Dѡ������ȷ�ģ�ѡCD��
��ˣ�������ȷ���ǣ��ң�CD��
(2)װ��(��)��װ�о������ữ�ĸ��������Һ���ữ������������ӣ�����������Ȼ�����Һ��������ɫ����������˵���Ƕ�������������������ã�֤����ϩ���Ƿ���SO2�����Լ���Ʒ����Һ��
��ˣ�������ȷ���ǣ����ɿ��������ữ�ĸ��������Һ�б����ͺ���SO42-��ƿ����Һ����Ʒ����Һ��
(3)����������Һ�ɳ�����������������Ȼ�̼��Һ��ɫ��֤����ϩ�ܷ����ӳɷ�Ӧ����ˣ�Ϊ��ȥ�����������壬��װ��(��)��(��)֮������һ��װ������NaOH��Һ��ϴ��ƿ����װ��(��)����ʢ��������Ȼ�̼��Һ��ϴ��ƿ��������Ȼ�̼��Һ��ɫ��֤����ϩ�ܷ����ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽ��CH2=CH2+Br2��CH2Br-CH2Br��
��ˣ�������ȷ���ǣ�NaOH��Һ��������Ȼ�̼��Һ��CH2=CH2+Br2��CH2Br-CH2Br��