��Ŀ����
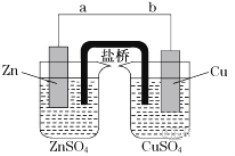
����Ŀ�������й����ĺ����ߣ����躣Ͽ��������������ˮ���ۺ����ô��п�Ϊ. ��ˮ���庬��ԼΪ65 mg��L��1���Ӻ�ˮ����ȡ��Ĺ���������ͼ��

(1)���ϲ�������ѻ������̬���壬������ֽ�֮ת��ɻ���̬���壬��Ŀ����______.
(2)�����ͨ���ȿ�����ˮ��������Br2�����������________��
A�������� B����ԭ�� C���ӷ��� D����ʴ��
(3)�������̢����漰�����ӷ�Ӧ���£��������淽���������ʵ��Ļ�ѧ��������
______Br2��______CO32-===______BrO3-��______Br����______CO2��
(4)���������д�������������Ҳ�����ö�������ˮ��Һ���գ�������������������.д�������������ˮ��Һ��Ӧ�Ļ�ѧ����ʽ��_____.
(5)ʵ���ҷ����廹�������ܼ���ȡ�������п������������ȡ������________.
A.�Ҵ� B.���Ȼ�̼ C.�ռ���Һ D.��
���𰸡�����(��Ũ��)��Ԫ�� C 3 3 1 5 3 SO2��Br2��2H2O===2HBr��H2SO4 BD
��������
��1��ֱ�ӰѺ�ˮ�е�Br��������Br2���õ������Ũ�Ⱥܵͣ�����ֱ�����ã������Ҫ��������ø�Ũ������Һ�����Բ����˲�����������̬����ת��ɻ���̬����IJ��衣��������(��Ũ��)��Ԫ�ء�
��2��������ͨ���ȿ�����ˮ��������Br2��˵�����ӷ����ʴ�ѡC��
��3���۲췴Ӧ�и�Ԫ�صĻ��ϼ۱仯��ֻ��Br�����仯���÷�ӦΪ�绯��Ӧ������������ƽ����3Br2��3CO32-=BrO3-��5Br����3CO2����
��4�������������һ����ԭ�ԣ���ɱ�����Ϊ�������Br2����ԭ��Br��,���ȱ����ƽ��ԭ���������ʽΪSO2��Br2��2H2O=2HBr��H2SO4��
��5����ȡ��ѡ���ԭ������������ȡ���е��ܽ��Ҫ��������ȡ����ˮ�����ܣ�������Щԭ���ֻ��BD��