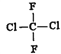��Ŀ����
����Ŀ���±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�

(1)д��Ԫ�آݵ�ԭ�ӹ����ʾʽ______________��
(2)д��Ԫ�آ�Ļ�̬ԭ�ӵļ۵����Ų�ʽ_________��
(3)��������ds����Ԫ����_________(����)
(4)Ԫ�آ��γɵĵ�������____���γɵľ��壬�þ����ȡ�Ķѻ���ʽ��_______�������Т�Ԫ��ԭ�ӵ���λ����_________��
(5)Ԫ���ܡ��ݡ��ޡ��ߵ����Ӱ뾶��С�����˳����_______(�����ӷ��ű�ʾ)
(6)д��Ԫ�آٺ�Ԫ�آ��γɻ�����ĵ���ʽ_________________��
(7)Ԫ�آ����ĵڶ������ֱܷ�Ϊ��I��=1753kJ/mo1��I��=1959kJ/mo1���ڶ�������I�����ԭ����____________________________��
(8)��ѧ�ҷ��֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص�����ͼ��ʾ��ͼ�Тڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ______(�ö�Ӧ��Ԫ�ط��ű�ʾ)��

���𰸡� Al��![]() ��[Ne]
��[Ne] ![]() 3d84s2 �� ���� �����������ܶѻ� 12 Al3+<Mg2+<Cl-<S2- K+[: H]- ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӡ� MgNi3C
3d84s2 �� ���� �����������ܶѻ� 12 Al3+<Mg2+<Cl-<S2- K+[: H]- ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӡ� MgNi3C
����������Ԫ�����ڱ����Եó�Ԫ����ΪH����ΪC����ΪO����ΪMg����ΪAl����ΪS����ΪCl����ΪK����ΪNi����ΪCu��
(1)Ԫ����ΪAl��ԭ�ӹ����ʾʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��Ԫ����ΪNi����̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2���ʴ�Ϊ��3d84s2��
(3) ds����Ԫ�ص���B����B��Ϊ��11�е�12��Ԫ�أ���Ԫ�������ڱ��е�λ�ÿ�֪�������ڵ�11�У�Ϊ�����壬����ds��Ԫ�أ��ʴ�Ϊ������
(4)Ԫ����ΪCu���γɵĵ������ɽ������γɵľ��壬�þ����ȡ�Ķѻ���ʽ�������������ܶѻ�����������Ԫ��ԭ�ӵ���λ����12���ʴ�Ϊ�������������������ܶѻ��� 12��
(5)һ����ԣ����ӵĵ��Ӳ���Խ�࣬�����Ӱ뾶Խ���Ӳ�ṹ��ͬ�����ӣ��˵����Խ�࣬���Ӱ뾶ԽС��Ԫ���������������������Ӱ뾶��С�����˳����Al3+<Mg2+<Cl-<S2-���ʴ�Ϊ��Al3+<Mg2+<Cl-<S2-��
(6)Ԫ����ΪH��Ԫ����ΪK���γɻ�����Ϊ���ӻ��������ʽΪK+[: H]-���ʴ�Ϊ��K+[: H]-��
(7) �ڶ������ܣ�ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӣ����µڶ�������I��<I�����ʴ�Ϊ��ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӣ�
��8�������������ֱ�λ�ھ��������ġ����㡢���ģ�Cԭ�Ӹ���Ϊ1��Mgԭ�Ӹ���Ϊ8��![]() =1��Niԭ�Ӹ���Ϊ6��
=1��Niԭ�Ӹ���Ϊ6��![]() =3����ѧʽΪMgNi3C���ʴ�Ϊ��MgNi3C��
=3����ѧʽΪMgNi3C���ʴ�Ϊ��MgNi3C��

 ����5��2���ϵ�д�
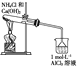
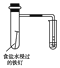
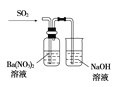
����5��2���ϵ�д�����Ŀ������ʵ��װ��������Ӧ������ȷ����(����)
ѡ�� | A | B | C | D |
װ�� |
|
|
|
|
���� | ��֤��Al(OH)3�����ڰ�ˮ | ��֤���ǽ����ԣ�Cl>C>Si | ��֤���������ⸯʴ | ϴ��ƿ�в����İ�ɫ������BaSO3 |
A. A B. B C. C D. D