��Ŀ����
��12�֣���1��H+����H2O�γ�H3O+��H3O+��Oԭ�Ӳ���  �ӻ�������DZ�ˮ���� �����С������
�ӻ�������DZ�ˮ���� �����С������
��2���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ʾ��ͼ��δ��˳�������������־����о���������ͬ���� ������д��ţ���

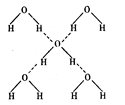
��3���ڱ��ľ����У�ÿ��ˮ���������ڵ� 4 ��ˮ�����γ��������ͼ��ʾ������֪���������ȣ��� 1 mol ˮ�ɱ�ת��Ϊˮ����������������51KJ/mol��������⣬ˮ���Ӽ仹���ڷ��»�����11 kJ / mol �� , �������������ġ����ܡ����ƻ� lmol ��������������Ϊ ����ġ����ܡ����� kJ / mol ��
����ġ����ܡ����� kJ / mol ��
��4��CaO��NaCl�ľ���ͬΪ���������ṹ����֪CaO�����ܶ�Ϊag��cm-3�� ��ʾ�����ӵ���������CaO�������Ϊ cm3��
��ʾ�����ӵ���������CaO�������Ϊ cm3��
��5����������Һ��,������������ԭ��Ӧ�б������뱻��ԭ�ĵ�Ԫ�ص����ʵ���֮��Ϊ2:5,
��KBiO3+��MnSO4+��H2SO4==��Bi2(SO4)3+�� +�� +��
д��Biͬ�����������Ԫ�صĵ����Ų�ʽ��
 �ӻ�������DZ�ˮ���� �����С������
�ӻ�������DZ�ˮ���� �����С��������2���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ʾ��ͼ��δ��˳�������������־����о���������ͬ���� ������д��ţ���

��3���ڱ��ľ����У�ÿ��ˮ���������ڵ� 4 ��ˮ�����γ��������ͼ��ʾ������֪���������ȣ��� 1 mol ˮ�ɱ�ת��Ϊˮ����������������51KJ/mol��������⣬ˮ���Ӽ仹���ڷ��»�����11 kJ / mol �� , �������������ġ����ܡ����ƻ� lmol ��������������Ϊ
 ����ġ����ܡ����� kJ / mol ��
����ġ����ܡ����� kJ / mol ��
��4��CaO��NaCl�ľ���ͬΪ���������ṹ����֪CaO�����ܶ�Ϊag��cm-3��
 ��ʾ�����ӵ���������CaO�������Ϊ cm3��
��ʾ�����ӵ���������CaO�������Ϊ cm3����5����������Һ��,������������ԭ��Ӧ�б������뱻��ԭ�ĵ�Ԫ�ص����ʵ���֮��Ϊ2:5,
��KBiO3+��MnSO4+��H2SO4==��Bi2(SO4)3+�� +�� +��
д��Biͬ�����������Ԫ�صĵ����Ų�ʽ��
��12�֣���1�� SP3 �� ����1�֣� ��2��BC ��2�֣�
SP3 �� ����1�֣� ��2��BC ��2�֣�
��3��20 ��2�֣���4�� ��2�֣�
��5��10KBiO3+4MnSO4+14H2SO4==5Bi2(SO4)3+4KMnO4+14H2O+3K2SO4 (2��)
1s22s22p63s23p63d104s24p3 (2��)
 SP3 �� ����1�֣� ��2��BC ��2�֣�
SP3 �� ����1�֣� ��2��BC ��2�֣� ��3��20 ��2�֣���4�� ��2�֣�
��5��10KBiO3+4MnSO4+14H2SO4==5Bi2(SO4)3+4KMnO4+14H2O+3K2SO4 (2��)
1s22s22p63s23p63d104s24p3 (2��)
��

��ϰ��ϵ�д�
�����Ŀ



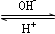 R(OH)3
R(OH)3  ��
��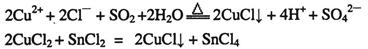
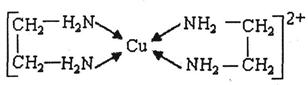
 ��
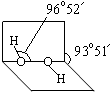
�� �е�ԭ�ӹ�����ӻ�����Ϊ ���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_ ��
�е�ԭ�ӹ�����ӻ�����Ϊ ���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����_ ��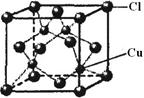
 (��ԭ�Ӽ�����������������ģ������ǻ����ʼ�����ȫ��ͬ)
(��ԭ�Ӽ�����������������ģ������ǻ����ʼ�����ȫ��ͬ) 

