��Ŀ����
����Ŀ���廯����һ����Ҫ���廯��������������ֽ��������������ȣ���ʯ���顢Һ�弰����Ϊԭ���Ʊ�CaBr22H2O��ʵ���������£�
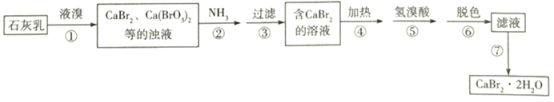
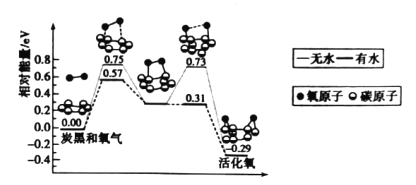
(1)�������������70�����£��¶Ȳ��˹��ߵ�ԭ����________________��
(2)����ʵ������ȡ����NH3�ķ�����ȷ����_____________�����ţ���
A�� B��
B�� C��
C��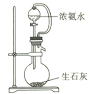 D��
D��
(3)��֪NH3������ΪN2��������з�����Ӧ�Ļ�ѧ����ʽΪ_______________________��
(4)����ܡ��ݵ�Ŀ��������_______________________________��________________________��
(5)�������_________________������ɫ���������˸����ʵ�____________________��
(6)����ߵõ���Ʒ�IJ���������__________________________________________��
���𰸡�Һ���ӷ� C Ca(BrO3)2+4NH3=CaBr2+2N2+6H2O ������� �к���Һ�е�Ca(OH)2��ʹ��Һ�������� ����̿ ������ ����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ�������
��������
����ʵ�����̣���֪ʯ������Һ�巢����Ӧ����CaBr2��Ca(BrO3)2�ȵ���Һ����������ͨ��NH3�����˺�õ���CaBr2����Һ�����������ԭ��Ӧԭ����д������ڷ����Ļ�ѧ��Ӧ����ʽ�����ʵ�������еĻ�ѧ���ʣ�������Һ�п��ܴ��ڵ����ʡ�ͨ�����ȡ����������ᡢ��ɫ�Ȳ��裬�������ܳ�ȥ����Һ�е��������ࡣ������յõ��IJ�Ʒ����������ߵIJ���������
(1)����Һ����������ʽ��н��Һ���ӷ������¶ȹ��ߣ���ӿ�Һ��Ļӷ������¶Ȳ��˹��ߣ���Ϊ��Һ���ӷ���
(2)A��NH4Cl�ֽ����NH3��HCl��NH3��HCl�ֻᷢ����Ӧ����NH4Cl��A�����
B��B����װ���Թܿ�Ӧ��������б����ֹ����ˮ������B�����
C��C����װ�ý�Ũ��ˮ������ʯ���п��Կ����Ƶð�����C����ȷ��
D��NaOH��Һ��NH4Cl��Һ�ڲ�����ʱ��Ҫ����NH3H2O��D�����
����������C��������⣻��Ϊ��C��
(3)���������Ϣ��NH3������ΪN2���������ͼ�����ʵı仯��������ԭ��Ӧԭ������֪Ca(BrO3)2��NH3��ԭΪCaBr2���ݴ˿�֪��Ӧ����ʽΪ��Ca(BrO3)2+4NH3=CaBr2+2N2+6H2O����Ϊ��Ca(BrO3)2+4NH3=CaBr2+2N2+6H2O��
(4)NH3��������ˮ�γ�NH3H2O����Ӧ�����Һ�к��й�����NH3H2O��ͨ�����ȵķ�������ʹNH3����Һ�����ݳ������������̣����˺����õĺ�CaBr2����Һ���ܺ���δ��Ӧ���Ca(OH)2��������������ᣬ������к͵�Ca(OH)2ʹ��Һ�������ԣ�������Һ�п��ܴ��ڵ����ʡ���Ϊ�������ˮ���к���Һ�е�Ca(OH)2��ʹ��Һ�������ԣ�
(5)����̿���ɡ���ף����нϴ�ı��������������һЩ��ɫ���ʶ�ʹ֮ʧȥԭ������ɫ���ʻ���̿��������ɫ������Ϊ������̿�������ԣ�
(6)����ߵõ��IJ�Ʒ���нᾧˮ���õ����ᾧˮ�����ʣ���ͨ������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ�������ķ�ʽ�õ����ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�