��Ŀ����
(14�֣��±���Ԫ�����ڱ���ǰ�����ڣ��ش��������⣺

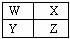
��1�������ڰ뵼����ϵ�Ԫ���� (��Ԫ�ط��ţ�������Ԫ�����ڱ��е�λ��Ϊ ��
��2��A��G����Ԫ�طֱ���EԪ�ض����γ����ֻ���������������ӻ�������� ��д��ѧʽ����ͬ�������ڹ��ۻ�������� �������ֻ������к��зǼ��Լ��Ļ������� ��
��3��������ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ���� ��������������������� ���û�ѧʽ��ʾ����
��4��ֻ����A��C����Ԫ�صĻ�������� ����Щ����������Է���������С���� ���û�������ӵĿռ乹���� ��
��5������Ԫ��H��ԭ�ӽṹʾ��ͼ ��Ԫ��H��Ԫ��J��ɵĻ������ˮ��Һ�м��������ռ���Һ����Ӧ�����ӷ���ʽΪ ��
��6����Ԫ��A��D��J��ɵļȺ������Ӽ��ֺ��й��ۼ��Ļ�����Ļ�ѧʽΪ ��

��1�������ڰ뵼����ϵ�Ԫ���� (��Ԫ�ط��ţ�������Ԫ�����ڱ��е�λ��Ϊ ��
��2��A��G����Ԫ�طֱ���EԪ�ض����γ����ֻ���������������ӻ�������� ��д��ѧʽ����ͬ�������ڹ��ۻ�������� �������ֻ������к��зǼ��Լ��Ļ������� ��
��3��������ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ���� ��������������������� ���û�ѧʽ��ʾ����
��4��ֻ����A��C����Ԫ�صĻ�������� ����Щ����������Է���������С���� ���û�������ӵĿռ乹���� ��
��5������Ԫ��H��ԭ�ӽṹʾ��ͼ ��Ԫ��H��Ԫ��J��ɵĻ������ˮ��Һ�м��������ռ���Һ����Ӧ�����ӷ���ʽΪ ��
��6����Ԫ��A��D��J��ɵļȺ������Ӽ��ֺ��й��ۼ��Ļ�����Ļ�ѧʽΪ ��
������ʽ2�֣�����ÿ��1�֣���14�֣�
(1) Si ��3���ڢ�A�� (2) Na2O �� Na2O2�� H2O��H2O2��H2O2��Na2O2
(3) NaOH Al(OH)3 (4) �� ���� ��������
(5) Al3++3OH-= Al(OH)3 �� ��6��NH4Cl
Al3++3OH-= Al(OH)3 �� ��6��NH4Cl
(1) Si ��3���ڢ�A�� (2) Na2O �� Na2O2�� H2O��H2O2��H2O2��Na2O2
(3) NaOH Al(OH)3 (4) �� ���� ��������
(5)
 Al3++3OH-= Al(OH)3 �� ��6��NH4Cl
Al3++3OH-= Al(OH)3 �� ��6��NH4Cl ��1�������ڰ뵼����ϵ�Ԫ����Si��λ�ڵ������ڵڢ�A��
��2��A��G����Ԫ�طֱ�H��Na��E��O�������������ӻ�������������ơ��������ơ����ڹ��ۻ��������ˮ��˫��ˮ���������ƺ�˫��ˮ�ж����зǼ��Լ���
��3��������Խǿ������������ˮ����ļ���Խǿ���ƵĽ�������ǿ�������������Ƶļ�����ǿ�����������������������
��4��A��C����Ԫ����̼���⣬�γɵĻ����������࣬��Щ����������Է���������С���Ǽ��飬��������������ṹ��
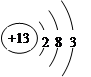
��5��H������������Ϊ13��ԭ�ӽṹʾ��ͼΪ ��J����Ԫ�أ��Ȼ����������������Ʒ�Ӧ��������������ɫ����������ʽΪAl3++3OH-= Al(OH)3 �� ��
��J����Ԫ�أ��Ȼ����������������Ʒ�Ӧ��������������ɫ����������ʽΪAl3++3OH-= Al(OH)3 �� ��
��6��Ԫ��A��D��J�ֱ���H��N��Cl����ɵļȺ������Ӽ��ֺ��й��ۼ��Ļ��������Ȼ�泥��仯ѧʽΪNH4Cl ��
��2��A��G����Ԫ�طֱ�H��Na��E��O�������������ӻ�������������ơ��������ơ����ڹ��ۻ��������ˮ��˫��ˮ���������ƺ�˫��ˮ�ж����зǼ��Լ���
��3��������Խǿ������������ˮ����ļ���Խǿ���ƵĽ�������ǿ�������������Ƶļ�����ǿ�����������������������
��4��A��C����Ԫ����̼���⣬�γɵĻ����������࣬��Щ����������Է���������С���Ǽ��飬��������������ṹ��
��5��H������������Ϊ13��ԭ�ӽṹʾ��ͼΪ
 ��J����Ԫ�أ��Ȼ����������������Ʒ�Ӧ��������������ɫ����������ʽΪAl3++3OH-= Al(OH)3 �� ��
��J����Ԫ�أ��Ȼ����������������Ʒ�Ӧ��������������ɫ����������ʽΪAl3++3OH-= Al(OH)3 �� ����6��Ԫ��A��D��J�ֱ���H��N��Cl����ɵļȺ������Ӽ��ֺ��й��ۼ��Ļ��������Ȼ�泥��仯ѧʽΪNH4Cl ��

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ
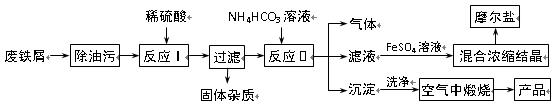 ��֪��FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3������Һ��
��֪��FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3������Һ��