��Ŀ����
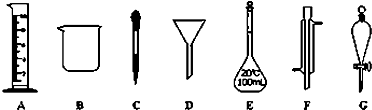
ʵ���ǻ�ѧ�о���һ����Ҫ�ֶΣ�������ͼ��ʾA~G���������������Ҫ����ա�
��1��д���������ƣ�B ��F
��2������ʵ��������õ�����G���� ��ѡ������ѡ��ı����ĸ����
a������ˮ��CC14�Ļ���� b������ˮ�;ƾ��Ļ���� c������ˮ����ɰ�Ļ����
��3��ʵ��������100mL 0.5mol/L��������Һ��
�����й�������E��ʹ�÷����У���ȷ���� ��ѡ������ѡ��ı����ĸ����
a��ʹ��ǰӦ����Ƿ�©Һ b��ʹ��ǰ������
c�������������ʷ�Ӧ���ܽ������ d������Һ��ֱ��ת�Ƶ�����ƿ��
����10mol/L��Ũ���� mL��ȡ�ø��������ʱ����Ҫ�õ����������е�A��
��ѡ�������ı����ĸ����
����ʵ��ʱ�������в��衰�������ȡ���ܽ��ת�ơ����ݡ����浽�Լ�ƿ�С��������ƣ����ݺ�Һ��λ�ã�������ͼ����ȷ���� ��ѡ������ѡ��ı����ĸ����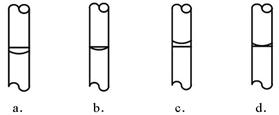
�������Լ�ƿ��ǩ����д��Ӧ���ݣ�ʢ���������ƺõ���Һ��[(��ǩ������ͼ)]��
��1���ձ��������ܣ�2��a ��3���� ac �� 5����5.0���� C �� d �� ���� 0.5mol/L
���������������1�����������Ĺ����֪������B���ձ���F�������ܡ�
��2��G�Ƿ�Һ©������Һʵ������Ҫ�õ���a��ȷ��ˮ�;ƾ����ܣ����ܷ�Һ��C�ǹ��ˣ���ѡa��
��3����E������ƿ��ʹ��ǰ��Ҫ��©��������Ҫ��ɣ�a��ȷ��b����ȷ�������������ʷ�Ӧ���ܽ��������c��ȷ������Һ��Ҫ��ȴ��ſ�ֱ��ת�Ƶ�����ƿ�У�d����ȷ����ѡac��
����10mol/L��Ũ����������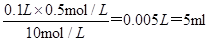 ��Ũ�������ȡ��Ҫ��Ͳ�ͽ�ͷ�ιܣ���ѡC��
��Ũ�������ȡ��Ҫ��Ͳ�ͽ�ͷ�ιܣ���ѡC��
�۶���ʱҺ�����͵�Ϳ̶������У���ѡd��
�ܱ�ǩ�ϵ����������������ᣬ������Ũ��0.5mol/L��
���㣺����������ʶ��һ�����ʵ���Ũ�ȵ�����
��������ѧʵ�鳣��������ʹ�÷����ͻ�ѧʵ����������ǽ��л�ѧʵ��Ļ������Ի�ѧʵ��Ŀ����벻����ѧʵ��Ļ������������Ը���������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������