��Ŀ����
ʵ���ǻ�ѧ�о���һ����Ҫ�ֶΣ�������ͼ��ʾA��G���������������Ҫ����գ�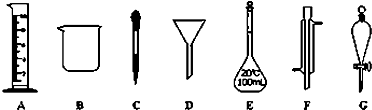
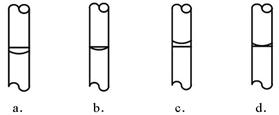
��1��д���������ƣ�D
��2������ʵ��������õ�����G����
a������ˮ��CCl4�Ļ���� b������ˮ�;ƾ��Ļ���� c������ˮ����ɰ�Ļ����
��3��ʵ��������100mL 0.5mol/L��������Һ��
�����й�������E��ʹ�÷����У���ȷ����
a��ʹ��ǰӦ����Ƿ�©Һ b��ʹ��ǰ������
c�������������ʷ�Ӧ���ܽ������ d������Һ��ֱ��ת�Ƶ�����ƿ��
������10mol/L��Ũ����
�����в�����ʹ���Ƶ���ҺŨ��ƫ�ߵ���
A��û�н�ϴ��Һת�Ƶ�����ƿ B��ת�ƹ�������������Һ����
C��ҡ�Ⱥ�Һ���½�������ˮ D������ʱ���ӿ̶��ߣ�
��������1������ͼʾ�������Ĺ���д�����������ƣ�
��2������GΪ��Һ©��������ȡ����Һ�����г�ʹ�÷�Һ©����
��3����EΪ����ƿ����������ƿ�Ĺ��켰��ȷʹ�÷������н��
�ڸ���n=cV������Ȼ�������ʵ������ٸ�����Һϡ���������ʵ����ʵ�������������Ҫ10mol/L��Ũ������������ȡŨ����ʱ��Ҫʹ����Ͳ�ͽ�ͷ�ιܣ�
�۸���c=
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
��2������GΪ��Һ©��������ȡ����Һ�����г�ʹ�÷�Һ©����
��3����EΪ����ƿ����������ƿ�Ĺ��켰��ȷʹ�÷������н��
�ڸ���n=cV������Ȼ�������ʵ������ٸ�����Һϡ���������ʵ����ʵ�������������Ҫ10mol/L��Ũ������������ȡŨ����ʱ��Ҫʹ����Ͳ�ͽ�ͷ�ιܣ�
�۸���c=
| n |
| V |
����⣺��1��D����������Ϊ©��������F������Ϊ�����ܣ�
�ʴ�Ϊ��©��������ͨ©�����������ܣ�
��2������GΪ��Һ©�����ڷ��뻥�����ܵĻ��Һ��ʱ��ʹ�õ���Һ©����������ƾ���ˮ�Ļ������Ҫ�������������ˮ����ɳ��Ҫͨ�����˲������������ж������õ���Һ©��������ֻ��a��ȷ��
�ʴ�Ϊ��a��
��3����EΪ100mL����ƿ��a������ƿ��ƿ�������ƹ�������Ҫҡ�ȣ�����ʹ��ǰӦ����Ƿ�©Һ������Ӱ�����ƽ������a��ȷ��
b������ʱ��Ҫ��������ˮ������ƿ������������ˮ��Ӱ���������ʵ�������Һ���������ʹ��ǰ����Ҫ��ɣ���b����
c������ƿֻ����������һ�����ʵ���Ũ�ȵ���Һ�������������ʷ�Ӧ���ܽ����������c��ȷ��
d������Һ���ƫ����ȴ����Һ��������С���������Ƶ���Һ���ƫС�����Բ��ܽ��ȵ���Һֱ��ת�Ƶ�����ƿ�У���d����
�ʴ�Ϊ��ac��
��100mL 0.5mol/L��������Һ�к����Ȼ�������ʵ���Ϊ0.05mol����Ҫ10mol/L��Ũ�������Ϊ��
=0.005L=5.0mL��ȡ�ø��������ʱ����Ҫ�õ����������е�A��Ͳ��C��ͷ�ιܣ�
�ʴ�Ϊ��5.0��C��
��A��û�н�ϴ��Һת�Ƶ�����ƿ���������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���A����
B��ת�ƹ�������������Һ�������������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���B����
C��ҡ�Ⱥ�Һ���½�������ˮ���������Ƶ���Һ���ƫ����Һ���ʵ���Ũ��ƫ�ͣ���C����
D������ʱ���ӿ̶��ߣ����¼��������ˮ���ƫС����Һ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ���D��ȷ��
��ѡD��
�ʴ�Ϊ��©��������ͨ©�����������ܣ�
��2������GΪ��Һ©�����ڷ��뻥�����ܵĻ��Һ��ʱ��ʹ�õ���Һ©����������ƾ���ˮ�Ļ������Ҫ�������������ˮ����ɳ��Ҫͨ�����˲������������ж������õ���Һ©��������ֻ��a��ȷ��
�ʴ�Ϊ��a��
��3����EΪ100mL����ƿ��a������ƿ��ƿ�������ƹ�������Ҫҡ�ȣ�����ʹ��ǰӦ����Ƿ�©Һ������Ӱ�����ƽ������a��ȷ��
b������ʱ��Ҫ��������ˮ������ƿ������������ˮ��Ӱ���������ʵ�������Һ���������ʹ��ǰ����Ҫ��ɣ���b����
c������ƿֻ����������һ�����ʵ���Ũ�ȵ���Һ�������������ʷ�Ӧ���ܽ����������c��ȷ��
d������Һ���ƫ����ȴ����Һ��������С���������Ƶ���Һ���ƫС�����Բ��ܽ��ȵ���Һֱ��ת�Ƶ�����ƿ�У���d����
�ʴ�Ϊ��ac��
��100mL 0.5mol/L��������Һ�к����Ȼ�������ʵ���Ϊ0.05mol����Ҫ10mol/L��Ũ�������Ϊ��
| 0.05mol |
| 10mol/L |
�ʴ�Ϊ��5.0��C��
��A��û�н�ϴ��Һת�Ƶ�����ƿ���������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���A����
B��ת�ƹ�������������Һ�������������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���B����
C��ҡ�Ⱥ�Һ���½�������ˮ���������Ƶ���Һ���ƫ����Һ���ʵ���Ũ��ƫ�ͣ���C����
D������ʱ���ӿ̶��ߣ����¼��������ˮ���ƫС����Һ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ���D��ȷ��
��ѡD��
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ��������������Ĺ��켰ʹ�÷�������Ŀ�ѶȲ�������ѵ�������������ע���������ķ�����

��ϰ��ϵ�д�
�����Ŀ