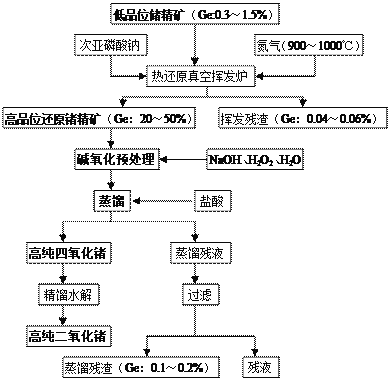
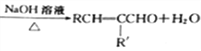��Ŀ����
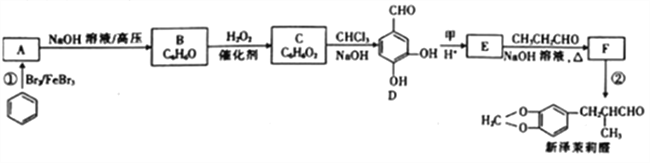
����Ŀ��A��B��C��D��E��F��ԭ���������������ǰ������Ԫ�ء�A�������к�����ḻ��Ԫ�أ�D��Eͬ���壬��E��ԭ��������D��������B��D��ɵĻ�������һ���������壻CԪ��ԭ�������P�ܼ���S�ܼ���1�����ӣ�Fԭ�ӵ�������������A��ͬ�����������Ӿ��������ݴ˻ش��������⡣
��1��FԪ���γɵĸۻ�̬���ӵĺ�������Ų�ʽΪ____________________________��
��2��E��һ�־���ǿ��ԭ�Ե���������ӵ�VSEPRģ��Ϊ____________________��
��3��C��D��EԪ�صĵ�һ�������ɴ�С����_________________������Ԫ�ط��ű�ʾ��
��4��A��D�γɵ�18���ӻ�����ɽ����Թ�ҵ��ˮ�е�CN-����Ϊ̼���κͰ�����Ӧ�����ӷ���ʽΪ___________________________��
��5��F��C�γɻ�����ľ�����ͼ��ʾ���û�����Ļ�ѧʽΪ__________��C���ӵ���λ����_________������������ı߳�Ϊa cm����þ����ܶ�Ϊ__________g/cm3��
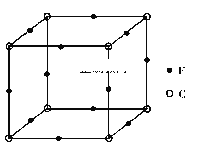
���𰸡� 1s22s22p63s23p63d9��[Ar]3d9 V�� N>O>S H2O2+CN-+OH- = CO32-+NH3 Cu3N 6 206/(a3NA)
��������A�������к�����ḻ��Ԫ�أ�ӦΪH��D��Eͬ���壬��E��ԭ��������D����������DΪO��EΪSԪ�أ�B��D��ɵĻ�������һ���������壬ӦΪ������̼��BΪCԪ�أ�CԪ��ԭ�������P�ܼ���S�ܼ���1�����ӣ���ԭ������С��O��ӦΪNԪ�أ�Fԭ�ӵ�������������A��ͬ����������������ӦΪCu��
(1)FΪCu���γɵĸۻ�̬���ӵĺ�������Ų�ʽΪ1s22s22p63s2sp63d9���ʴ�Ϊ��1s22s22p63s2sp63d9��[Ar]3d9��
(2)S��һ�־���ǿ��ԭ�Ե�������ΪSO2�������к���2�������µ��Ӷ�Ϊ(62��2/)2=1��Ϊsp2�ӻ�������ӵ�VSEPRģ��Ϊƽ�������Σ��ʴ�Ϊ��ƽ�������Σ�
(3)�ǽ�����Խǿ����һ������Խ��N��2p���Ӱ�����Ϊ�ȶ��ṹ�����һ��������С�����˳����N>O>S���ʴ�Ϊ��N>O>S��
(4)A��D�γɵ�18���ӻ�����ΪH2O2��Ϊ���Է�������CS2Ϊ�Ǽ��Է����������������ܹ��ɿ�֪H2O2������CS2���ɽ����Թ�ҵ��ˮ�е�CN����Ϊ̼���κͰ�����Ӧ�����ӷ���ʽΪH2O2+CN-+OH- = CO32-+NH3
(6)FΪCu��CΪN���ɾ���ʾ��ͼ��֪��Cuλ�ڶ�������ĿΪ8��1/8=1��NΪ������ĿΪ12��1/4=3����ѧʽΪCuN3��Cu���ӵ���λ����3��14��8=6��һ�������а�����һ��CuN3��һ������������Ϊ206/NA��һ�����������Ϊa3����ô�������ܶ�Ϊ206/(a3NA)���ʴ�Ϊ��CuN3��6.��206/(a3NA)